| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-05-05 15:49:56 UTC |
|---|
| Update Date | 2020-05-05 18:39:08 UTC |
|---|
| BMDB ID | BMDB0109687 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Tryptophan 2-C-mannoside |
|---|
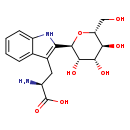
| Description | Tryptophan 2-C-mannoside, also known as 2'-α-D-mannosyltryptophan or C-man-TRP, belongs to the class of organic compounds known as indolyl carboxylic acids and derivatives. Indolyl carboxylic acids and derivatives are compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an indole ring. Based on a literature review a small amount of articles have been published on Tryptophan 2-C-mannoside. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1R)-1,5-Anhydro-1-{3-[(2S)-2-amino-2-carboxyethyl]-1H-indol-2-yl}-D-mannitol | ChEBI | | 2'-alpha-D-Mannosyl-L-tryptophan | ChEBI | | 2'-alpha-D-Mannosyltryptophan | ChEBI | | 2'-alpha-Mannosyltryptophan | ChEBI | | 2'-Tryptophan C-mannoside | ChEBI | | C(2)-alpha-D-Mannopyranosyl-L-tryptophan | ChEBI | | 2'-a-D-Mannosyl-L-tryptophan | Generator | | 2'-Α-D-mannosyl-L-tryptophan | Generator | | 2'-a-D-Mannosyltryptophan | Generator | | 2'-Α-D-mannosyltryptophan | Generator | | 2'-a-Mannosyltryptophan | Generator | | 2'-Α-mannosyltryptophan | Generator | | C(2)-a-D-Mannopyranosyl-L-tryptophan | Generator | | C(2)-Α-D-mannopyranosyl-L-tryptophan | Generator | | 2'-a-Mannosyl-L-tryptophan | HMDB | | 2'-Α-mannosyl-L-tryptophan | HMDB | | 2-(alpha-D-Mannopyranosyl)-L-tryptophan | HMDB | | 2-(Α-D-mannopyranosyl)-L-tryptophan | HMDB | | 2-alpha-D-Mannopyranosyl-L-tryptophan | HMDB | | 2-Α-D-mannopyranosyl-L-tryptophan | HMDB | | C2-alpha-D-Mannopyranosyltryptophan | HMDB | | C2-Α-D-mannopyranosyltryptophan | HMDB | | 2-(alpha-Mannopyranosyl)-L-tryptophan | HMDB | | 2-(Α-mannopyranosyl)-L-tryptophan | HMDB | | 2-alpha-D-Mannopyranosyltryptophan | HMDB | | 2-alpha-D-Mannosyl-L-tryptophan | HMDB | | 2-alpha-D-Mannosyltryptophan | HMDB | | 2-Α-D-mannopyranosyltryptophan | HMDB | | 2-Α-D-mannosyl-L-tryptophan | HMDB | | 2-Α-D-mannosyltryptophan | HMDB | | C-Glycosyltryptophan | HMDB | | C-Man-TRP | HMDB | | C-Mannosyl tryptophan | HMDB | | C-Mannosyltryptophan | HMDB | | L-Tryptophan 2-C-alpha-D-mannopyranoside | HMDB | | L-Tryptophan 2-C-mannopyranoside | HMDB | | L-Tryptophan 2-C-α-D-mannopyranoside | HMDB | | alpha-C-Man-TRP | HMDB | | Α-C-man-TRP | HMDB | | 2-(Mannopyranosyl)-tryptophan | MeSH | | 2(MP)-L-T | MeSH | | Tryptophan 2-C-mannoside | HMDB |
|
|---|
| Chemical Formula | C17H22N2O7 |
|---|
| Average Molecular Weight | 366.37 |
|---|
| Monoisotopic Molecular Weight | 366.142701056 |
|---|
| IUPAC Name | (2S)-2-amino-3-{2-[(2R,3S,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-1H-indol-3-yl}propanoic acid |
|---|
| Traditional Name | (2S)-2-amino-3-{2-[(2R,3S,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-1H-indol-3-yl}propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | N[C@@H](CC1=C(NC2=CC=CC=C12)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C17H22N2O7/c18-9(17(24)25)5-8-7-3-1-2-4-10(7)19-12(8)16-15(23)14(22)13(21)11(6-20)26-16/h1-4,9,11,13-16,19-23H,5-6,18H2,(H,24,25)/t9-,11+,13+,14-,15-,16+/m0/s1 |
|---|
| InChI Key | CPXSBHKDEPPWIX-RAYCSJGISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolyl carboxylic acids and derivatives. Indolyl carboxylic acids and derivatives are compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an indole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolyl carboxylic acids and derivatives |
|---|
| Direct Parent | Indolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolyl carboxylic acid derivative
- Hexose monosaccharide
- C-glycosyl compound
- Glycosyl compound
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- 3-alkylindole
- Indole
- Aralkylamine
- Benzenoid
- Substituted pyrrole
- Monosaccharide
- Oxane
- Heteroaromatic compound
- Pyrrole
- Secondary alcohol
- Amino acid
- Amino acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Dialkyl ether
- Ether
- Oxacycle
- Azacycle
- Monocarboxylic acid or derivatives
- Polyol
- Organic oxide
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Primary amine
- Primary alcohol
- Primary aliphatic amine
- Hydrocarbon derivative
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - CE-QTOF-MS system (Agilent 7100 CE + 6550 QTOF) 10V, Positive | splash10-014i-0049000000-429d66198fdcf1cabf9f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0109000000-fc7c1daf739549f05d41 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gxc-0926000000-eae85b92bb2b9afe0511 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o0-1930000000-291d7629de2d4ca7d6c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0095000000-c672ca30096473e70993 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0604-3289000000-1081bf67033725b64f12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-7930000000-6cf8b67a0c576645198f | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|