| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-26 13:18:14 UTC |
|---|
| Update Date | 2020-04-22 20:21:36 UTC |
|---|
| BMDB ID | BMDB0109610 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | SM(d18:1/26:1(17Z)) |
|---|
| Description | SM(d18:1/26:1(17Z)) is a sphingomyelin (SM). Sphingomyelins are members of the class of compounds known as sphingolipids (SPs), or glycosylceramides. SPs are lipids containing a backbone of sphingoid bases (e.g. sphingosine or sphinganine) that are often covalently bound to a fatty acid derivative through N-acylation. SPs are found in cell membranes, particularly in peripheral nerve cells and the cells found in the central nervous system (including the brain and spinal cord). Sphingolipids are extremely versatile molecules that have functions controlling fundamental cellular processes such as cell division, differentiation, and cell death. Impairments associated with sphingolipid metabolism are associated with many common human diseases such as diabetes, various cancers, microbial infections, diseases of the cardiovascular and respiratory systems, Alzheimer’s disease and other neurological syndromes. The biosynthesis and catabolism of sphingolipids involves a large number of intermediate metabolites where many different enzymes are involved. Simple sphingolipids, which include the sphingoid bases and ceramides, make up the early products of the sphingolipid synthetic pathways, while complex sphingolipids may be formed by the addition of head groups to the ceramide template (Wikipedia). SM is the major sphingolipid in mammals it is found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a phosphoethanolamine head group. In humans, sphingomyelin is the only membrane phospholipid not derived from glycerol. SM contains one polar head group, which is either phosphocholine or phosphoethanolamine. The plasma membrane of cells is highly enriched in sphingomyelin and is considered largely to be found in the exoplasmic leaflet of the cell membrane. However, there is some evidence that there may also be a sphingomyelin pool in the inner leaflet of the membrane. Moreover, neutral sphingomyelinase-2 - an enzyme that breaks down sphingomyelin into ceramide has been found to localise exclusively to the inner leaflet further suggesting that there may be sphingomyelin present there. Sphingomyelin can accumulate in a rare hereditary disease called Niemann-Pick Disease, types A and B. Niemann-Pick disease is a genetically-inherited disease caused by a deficiency in the enzyme sphingomyelinase, which causes the accumulation of sphingomyelin in spleen, liver, lungs, bone marrow, and the brain, causing irreversible neurological damage. SMs play a role in signal transduction. Sphingomyelins are synthesized by the transfer of phosphorylcholine from phosphatidylcholine to a ceramide in a reaction catalyzed by sphingomyelin synthase. In terms of its appearance and structure, SM(d18:1/26:1(17Z)) consists of an unsaturated 18-carbon sphingoid base with an attached unsaturated 17Z-hexacosenoyl fatty acid side chain. In most mammalian SPs, the 18-carbon sphingoid bases are predominant (PMID: 9759481 ). |
|---|
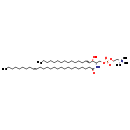
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| C26:1 Sphingomyelin | ChEBI | | N-(17Z-Hexacosenoyl)-sphing-4-enine-1-phosphocholine | ChEBI | | N-(Tricosanoyl)-sphing-4-enine-1-phosphocholine | HMDB | | Sphingomyelin | HMDB | | N-(17Z-Hexacosenoyl)-1-phosphocholine-sphing-4-enine | MetBuilder | | Sphingomyelin(D18:1/26:1(17Z)) | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-sphingosine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-D-erythro-sphingosine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-4-sphingenine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-D-sphingosine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-sphingenine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-erythro-4-sphingenine | MetBuilder | | SM(D18:1/26:1(17Z)) | ChEBI | | N-(17Z-Hexacosenoyl)-1-phosphocholine-sphinganine | MetBuilder | | Sphingomyelin(D18:0/26:1(17Z)) | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-dihydrosphingosine | MetBuilder | | N-(17Z-Hexacosenoyl)-1-phosphocholine-D-erythro-sphinganine | MetBuilder |
|
|---|
| Chemical Formula | C49H97N2O6P |
|---|
| Average Molecular Weight | 841.278 |
|---|
| Monoisotopic Molecular Weight | 840.708425358 |
|---|
| IUPAC Name | (2-{[(2S,3R,4E)-2-[(17Z)-hexacos-17-enamido]-3-hydroxyoctadec-4-en-1-yl phosphono]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | (2-{[(2S,3R,4E)-2-[(17Z)-hexacos-17-enamido]-3-hydroxyoctadec-4-en-1-yl phosphono]oxy}ethyl)trimethylazanium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCC\C=C\[C@@H](O)[C@H](COP([O-])(=O)OCC[N+](C)(C)C)NC(=O)CCCCCCCCCCCCCCC\C=C/CCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C49H97N2O6P/c1-6-8-10-12-14-16-18-20-21-22-23-24-25-26-27-28-29-31-33-35-37-39-41-43-49(53)50-47(46-57-58(54,55)56-45-44-51(3,4)5)48(52)42-40-38-36-34-32-30-19-17-15-13-11-9-7-2/h20-21,40,42,47-48,52H,6-19,22-39,41,43-46H2,1-5H3,(H-,50,53,54,55)/b21-20-,42-40+/t47-,48+/m0/s1 |
|---|
| InChI Key | YXEXWUZHFGYOHJ-UOJCCMJYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphosphingolipids. These are sphingolipids with a structure based on a sphingoid base that is attached to a phosphate head group. They differ from phosphonospingolipids which have a phosphonate head group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Phosphosphingolipids |
|---|
| Direct Parent | Phosphosphingolipids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sphingoid-1-phosphate or derivatives
- Phosphocholine
- Phosphoethanolamine
- Dialkyl phosphate
- Fatty amide
- N-acyl-amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Carboxylic acid derivative
- Organic zwitterion
- Alcohol
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Amine
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Axon
- Cell membrane
- Endosome
- Membrane
- Myelin sheath
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-053l-6011109040-78d43e222f56260422fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0536-2142109100-86de838a540453f018fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001s-9166002300-ec56b7022d6302efcb2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000490-cb91e2cdfa0fc1ef0e53 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2015004920-8dd85b3408ce46984d72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9128002000-1f1df5bc5b2e61edb1da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000001190-852973a8181afd58aa19 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dk-0000009290-2a89ec676a087342ac72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0000009110-41d912ffebeab2f9e19c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000x-0600000090-f3afb84c3623381e151f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000x-0600000090-f3afb84c3623381e151f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0900000030-ea80282a636c1f91d6e7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000090-dbddecc32b1e8eef807d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000000390-a19ee8177a5c774ca039 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9001030100-33cf30aa4edf3e21f80c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000001090-b6e3615b7d0d42bfc294 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03f0-0000007090-7266def15f5db339d557 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0000009020-fbc8eda2d3b38d2ae7b9 | View in MoNA |
|---|
|
|---|