| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-26 13:17:56 UTC |
|---|
| Update Date | 2020-04-22 20:21:35 UTC |
|---|
| BMDB ID | BMDB0109606 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Pentanoyl-CoA |
|---|
| Description | Pentanoyl-CoA belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. Pentanoyl-CoA is a strong basic compound (based on its pKa). pentanoyl-CoA can be biosynthesized from 3-hydroxyvalproic acid CoA; which is catalyzed by the enzyme trifunctional enzyme, mitochondrial. In humans, pentanoyl-CoA is involved in valproic acid metabolism pathway. This is then used in the citric acid cycle to start a chain of reactions, eventually forming many adenosine triphosphates. Pentanoyl CoA is an acyl-CoA with the C-5 Acyl chain. The second step, transfer of the acyl group to CoA (the same molecule that carries acetyl groups as acetyl-CoA), conserves free energy in the formation of a thioester bond. Because there is no transport protein for CoA adducts, acyl groups must enter the mitochondria via a shuttle system involving the small molecule carnitine. |
|---|
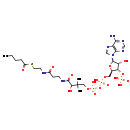
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pentanoyl-coenzyme A | HMDB |
|
|---|
| Chemical Formula | C26H44N7O17P3S |
|---|
| Average Molecular Weight | 851.651 |
|---|
| Monoisotopic Molecular Weight | 851.172723243 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-(pentanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-({[hydroxy([hydroxy(3-hydroxy-2,2-dimethyl-3-[(2-{[2-(pentanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy)phosphoryl]oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C26H44N7O17P3S/c1-4-5-6-17(35)54-10-9-28-16(34)7-8-29-24(38)21(37)26(2,3)12-47-53(44,45)50-52(42,43)46-11-15-20(49-51(39,40)41)19(36)25(48-15)33-14-32-18-22(27)30-13-31-23(18)33/h13-15,19-21,25,36-37H,4-12H2,1-3H3,(H,28,34)(H,29,38)(H,42,43)(H,44,45)(H2,27,30,31)(H2,39,40,41)/t15-,19-,20-,21?,25-/m1/s1 |
|---|
| InChI Key | RXUATCUKICAIOA-TVCSPYKZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous metal compounds |
|---|
| Class | Homogeneous transition metal compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous transition metal compounds |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | - Homogeneous transition metal
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-2901000120-2a423c1bd81049be2f37 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1913000000-a7882da7f4659358dd99 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1911000000-dd4d2426d295e6cea710 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-7930140450-c93bb9584e9108db8543 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-6910010000-390528f5d6ef7539cb68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-5900100000-f9c4115d1b808c86f8f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000000090-3758b512fc2398854707 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900001560-656412ccb7e988988a2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0029000000-298400882ff5c02d176c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000090-c1d8a32a3fcbde2b3b71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fas-9200202360-645cab450d162098ff13 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p0-6204702920-60948d54a96619a917a8 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|