| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:44 UTC |
|---|
| Update Date | 2020-03-13 22:44:02 UTC |
|---|
| BMDB ID | BMDB0096254 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | p-Coumaric acid sulfate |
|---|
| Description | p-Coumaric acid sulfate, also known as p-(sulfooxy)-cinnamic acid or zosteric acid, belongs to the class of organic compounds known as cinnamic acids. These are organic aromatic compounds containing a benzene and a carboxylic acid group forming 3-phenylprop-2-enoic acid. Based on a literature review a significant number of articles have been published on p-Coumaric acid sulfate. |
|---|
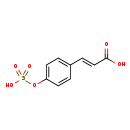
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(Sulfooxy)benzeneacrylic acid | ChEBI | | p-(Sulfooxy)-cinnamic acid | ChEBI | | p-(Sulphooxy)-cinnamic acid | ChEBI | | Zosteric acid | ChEBI | | 4-(Sulfooxy)benzeneacrylate | Generator | | 4-(Sulphooxy)benzeneacrylate | Generator | | 4-(Sulphooxy)benzeneacrylic acid | Generator | | p-(Sulfooxy)-cinnamate | Generator | | p-(Sulphooxy)-cinnamate | Generator | | Zosterate | Generator | | p-Coumarate sulfate | Generator | | p-Coumarate sulphate | Generator | | p-Coumaric acid sulfuric acid | Generator | | p-Coumaric acid sulphuric acid | Generator | | (2E)-3-[4-(Sulfooxy)phenyl]prop-2-enoate | HMDB | | (2E)-3-[4-(Sulphooxy)phenyl]prop-2-enoate | HMDB | | (2E)-3-[4-(Sulphooxy)phenyl]prop-2-enoic acid | HMDB | | p-Sulfoxycinnamic acid | HMDB | | p-Sulfoxycinnamate | HMDB | | p-Sulphoxycinnamate | HMDB | | p-Sulphoxycinnamic acid | HMDB | | (e)-3-(4-Sulfooxyphenyl)prop-2-enoate | HMDB | | (e)-3-(4-Sulphooxyphenyl)prop-2-enoate | HMDB | | (e)-3-(4-Sulphooxyphenyl)prop-2-enoic acid | HMDB | | (2E)-3-[4-(Sulfooxy)phenyl]-2-propenoic acid | HMDB | | (2E)-3-[4-(Sulfooxy)phenyl]prop-2-enoic acid | HMDB | | 3-[4-(Sulfooxy)phenyl]-2-propenoic acid | HMDB | | 4-Hydroxycinnamic acid sulfate | HMDB | | 4-Hydroxycinnamic acid sulphate | HMDB | | Coumaric acid sulfate | HMDB | | Coumaric acid sulphate | HMDB | | Coumaric acid-O-sulfate | HMDB | | Coumaric acid-O-sulphate | HMDB | | p-Coumaric acid sulphate | HMDB | | p-trans-Coumaric acid sulfate | HMDB | | p-trans-Coumaric acid sulphate | HMDB | | p-Coumaric acid sulfate | HMDB |
|
|---|
| Chemical Formula | C9H8O6S |
|---|
| Average Molecular Weight | 244.22 |
|---|
| Monoisotopic Molecular Weight | 244.004159152 |
|---|
| IUPAC Name | (2E)-3-[4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| Traditional Name | (2E)-3-[4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)\C=C\C1=CC=C(OS(O)(=O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H8O6S/c10-9(11)6-3-7-1-4-8(5-2-7)15-16(12,13)14/h1-6H,(H,10,11)(H,12,13,14)/b6-3+ |
|---|
| InChI Key | OYDCCWNLILCHDJ-ZZXKWVIFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cinnamic acids. These are organic aromatic compounds containing a benzene and a carboxylic acid group forming 3-phenylprop-2-enoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Cinnamic acids |
|---|
| Direct Parent | Cinnamic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid
- Phenylsulfate
- Arylsulfate
- Phenoxy compound
- Styrene
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Organic sulfuric acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00g0-7391000000-bc201a71a132975def11 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02du-1930000000-3d315c3620eb789149d8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-03dl-0940000000-fbb46379c85b55e3ed0c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-014i-0900000000-c807c3402a792fb5c9c7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-014i-0900000000-7dfb031dd66f2f93fd55 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-03dl-0940000000-2fc6870eecc42a60801c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0190000000-19dd364ad88ddbb9db31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0940000000-2b3b871ba7a5cf24decc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-9500000000-af136c2da6ca0b33e4da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0190000000-cc7126b9fa36790786b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-044m-0940000000-1b9d6de3829c9378ebde | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-2900000000-0ec900596fffebf912a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-e0264036c945f3b958c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0490000000-d929eb453a459e4f242f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-5900000000-eb9f4cc9a4bbe250928e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0190000000-faaabde50e3c72325b25 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054k-0490000000-c85b169c2a0a99e13927 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fc0-0900000000-e7d68ea3024fdbaf3b44 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|