| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:42 UTC |
|---|
| Update Date | 2020-03-13 22:44:01 UTC |
|---|
| BMDB ID | BMDB0096252 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
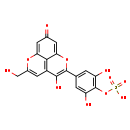
| Common Name | {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium |
|---|
| Description | {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium, also known as ({7-[3,5-dihydroxy-4-(sulphooxy)phenyl]-6-hydroxy-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl}methyl)oxidanium, belongs to the class of organic compounds known as 3-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-3 position of the flavonoid skeleton. Based on a literature review very few articles have been published on {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenoxy}dihydroxyoxo-λ⁶-sulphanylium | Generator | | ({7-[3,5-dihydroxy-4-(sulphooxy)phenyl]-6-hydroxy-11-oxo-2,8-dioxatricyclo[7.3.1.0⁵,¹³]trideca-1(12),3,5(13),6,9-pentaen-3-yl}methyl)oxidanium | HMDB |
|
|---|
| Chemical Formula | C18H12O11S |
|---|
| Average Molecular Weight | 436.34 |
|---|
| Monoisotopic Molecular Weight | 436.01003238 |
|---|
| IUPAC Name | {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0^{5,13}]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenyl}oxidanesulfonic acid |
|---|
| Traditional Name | {2,6-dihydroxy-4-[4-hydroxy-7-(hydroxymethyl)-11-oxo-2,8-dioxatricyclo[7.3.1.0^{5,13}]trideca-1(12),3,5(13),6,9-pentaen-3-yl]phenyl}oxidanesulfonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OCC1=CC2=C3C(O1)=CC(=O)C=C3OC(=C2O)C1=CC(O)=C(OS(O)(=O)=O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C18H12O11S/c19-6-9-5-10-15-13(27-9)3-8(20)4-14(15)28-17(16(10)23)7-1-11(21)18(12(22)2-7)29-30(24,25)26/h1-5,19,21-23H,6H2,(H,24,25,26) |
|---|
| InChI Key | GKLJHKNETPWJSD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-3 position of the flavonoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Hydroxyflavonoids |
|---|
| Direct Parent | 3-hydroxyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-hydroxyflavonoid
- 3'-hydroxyflavonoid
- Phenylsulfate
- Arylsulfate
- 1-benzopyran
- Benzopyran
- Phenoxy compound
- Resorcinol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Heteroaromatic compound
- Organic sulfuric acid or derivatives
- Cyclic ketone
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0000900000-591eebb6efa92e86b1fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-0009700000-829e13186efe1f04b22d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2983100000-ecbf1afd59d3fbcfab48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-6b0a52071da29f7cf450 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pbi-0009400000-360327c96d4473d1358b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ea-5249000000-52c29bbfae0d8e29c06c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-83bcc9d5549fb76ae714 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f79-0000900000-6b31930cea96c8ecb447 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-6797300000-1df616a8eec84e323ad3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-deafe8451b439866b1a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0570-0029000000-6427b1b682560e123b8d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zi9-0497100000-4003246b4d5d8743d15b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|