| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:22 UTC |
|---|
| Update Date | 2020-04-22 18:56:53 UTC |
|---|
| BMDB ID | BMDB0096232 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
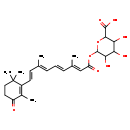
| Common Name | 4-Ketoretinoic acid glucuronide |
|---|
| Description | 4-Ketoretinoic acid glucuronide belongs to the class of organic compounds known as diterpene glycosides. These are diterpenoids in which an isoprene unit is glycosylated. 4-Ketoretinoic acid glucuronide is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Ketoretinoate glucuronide | Generator |
|
|---|
| Chemical Formula | C26H34O9 |
|---|
| Average Molecular Weight | 490.5428 |
|---|
| Monoisotopic Molecular Weight | 490.220282686 |
|---|
| IUPAC Name | 6-{[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-3-oxocyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoyl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | 6-{[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-3-oxocyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoyl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C\C(\C=C\C1=C(C)C(=O)CCC1(C)C)=C/C=C/C(/C)=C/C(=O)OC1OC(C(O)C(O)C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C26H34O9/c1-14(9-10-17-16(3)18(27)11-12-26(17,4)5)7-6-8-15(2)13-19(28)34-25-22(31)20(29)21(30)23(35-25)24(32)33/h6-10,13,20-23,25,29-31H,11-12H2,1-5H3,(H,32,33)/b8-6+,10-9+,14-7+,15-13+ |
|---|
| InChI Key | SIKFAVWPHMSCBL-FRCNGJHJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpene glycosides. These are diterpenoids in which an isoprene unit is glycosylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Diterpene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpene glycoside
- Retinoid ester
- Diterpenoid
- Retinoid skeleton
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- Beta-hydroxy acid
- Fatty acid ester
- Cyclohexenone
- Fatty acyl
- Dicarboxylic acid or derivatives
- Pyran
- Oxane
- Hydroxy acid
- Monosaccharide
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Cyclic ketone
- Carboxylic acid ester
- Secondary alcohol
- Ketone
- Polyol
- Carboxylic acid
- Organoheterocyclic compound
- Carboxylic acid derivative
- Oxacycle
- Acetal
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0adi-9121400000-fa740a33d3378521c3aa | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-6492038000-064f001b4fc21ffe7853 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r2-0693500000-1c93a2e712f7e42db4e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1981000000-d38a0a0c24fe50653deb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f72-2930000000-12402f7644c1817f4597 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0193400000-e632d10717d4280b92e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-4956200000-094ffc123fe6e74beb6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dm-9663000000-20ba0e60cb7315df7efd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0001900000-a8c9368c7ac385b0a49e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0170-1292200000-4b85dc33d73da2e7b002 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0r00-4951100000-e95621c87c00e0b108f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0452900000-e315d43e7a3da636ee90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-102i-0890100000-398a7bc32d6c4cf8fd54 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0921000000-9319c1f475566e5263d8 | View in MoNA |
|---|
|
|---|