| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:57 UTC |
|---|
| Update Date | 2020-04-22 18:56:44 UTC |
|---|
| BMDB ID | BMDB0096207 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Sinapic acid 4-O-sulfate |
|---|
| Description | Sinapic acid 4-O-sulfate, also known as (e)-sinapic acid sulphate or sinapate 4-O-sulfate, belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. Based on a literature review a small amount of articles have been published on Sinapic acid 4-O-sulfate. |
|---|
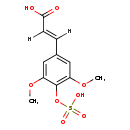
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sinapate 4-O-sulfate | Generator | | Sinapate 4-O-sulphate | Generator | | Sinapic acid 4-O-sulfuric acid | Generator | | Sinapic acid 4-O-sulphuric acid | Generator | | (2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid sulfate | HMDB | | (2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid sulphate | HMDB | | (e)-3,5-Dimethoxy-4-hydroxycinnamic acid sulfate | HMDB | | (e)-3,5-Dimethoxy-4-hydroxycinnamic acid sulphate | HMDB | | (e)-3-(4-Hydroxy-3,5-dimethoxyphenyl)acrylic acid sulfate | HMDB | | (e)-3-(4-Hydroxy-3,5-dimethoxyphenyl)acrylic acid sulphate | HMDB | | (e)-Sinapic acid sulfate | HMDB | | (e)-Sinapic acid sulphate | HMDB | | 3,5-Dimethoxy-4-hydroxy-trans-cinnamic acid sulfate | HMDB | | 3,5-Dimethoxy-4-hydroxy-trans-cinnamic acid sulphate | HMDB | | 3,5-Dimethoxy-4-hydroxycinnamic acid sulfate | HMDB | | 3,5-Dimethoxy-4-hydroxycinnamic acid sulphate | HMDB | | 3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid sulfate | HMDB | | 3-(4-Hydroxy-3,5-dimethoxyphenyl)-2-propenoic acid sulphate | HMDB | | 4-Hydroxy-3,5-dimethoxycinnamic acid sulfate | HMDB | | 4-Hydroxy-3,5-dimethoxycinnamic acid sulphate | HMDB | | e-Sinapinic acid sulfate | HMDB | | e-Sinapinic acid sulphate | HMDB | | Sinapic acid sulfate | HMDB | | Sinapic acid sulphate | HMDB | | Sinapic acid-4'-O-sulfate | HMDB | | Sinapic acid-4'-O-sulphate | HMDB | | Sinapic acid-4-O-sulfate | HMDB | | Sinapic acid-4-O-sulphate | HMDB | | Sinapic acid-4’-O-sulfate | HMDB | | Sinapic acid-4’-O-sulphate | HMDB | | Sinapinic acid sulfate | HMDB | | Sinapinic acid sulphate | HMDB | | Synapitic acid sulfate | HMDB | | Synapitic acid sulphate | HMDB | | trans-4-Hydroxy-3,5-dimethoxycinnamic acid sulfate | HMDB | | trans-4-Hydroxy-3,5-dimethoxycinnamic acid sulphate | HMDB | | trans-Sinapic acid sulfate | HMDB | | trans-Sinapic acid sulphate | HMDB | | trans-Sinapinic acid sulfate | HMDB | | trans-Sinapinic acid sulphate | HMDB |
|
|---|
| Chemical Formula | C11H12O8S |
|---|
| Average Molecular Weight | 304.27 |
|---|
| Monoisotopic Molecular Weight | 304.02528852 |
|---|
| IUPAC Name | (2E)-3-[3,5-dimethoxy-4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| Traditional Name | (2E)-3-[3,5-dimethoxy-4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(=C(\[H])C1=CC(OC)=C(OS(O)(=O)=O)C(OC)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H12O8S/c1-17-8-5-7(3-4-10(12)13)6-9(18-2)11(8)19-20(14,15)16/h3-6H,1-2H3,(H,12,13)(H,14,15,16)/b4-3+ |
|---|
| InChI Key | KJWQVTFGBFEXMV-ONEGZZNKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid
- Coumaric acid or derivatives
- Phenylsulfate
- Arylsulfate
- M-dimethoxybenzene
- Dimethoxybenzene
- Anisole
- Methoxybenzene
- Styrene
- Phenol ether
- Phenoxy compound
- Alkyl aryl ether
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Organic sulfuric acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Ether
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-01vo-1910000000-c5c23a198fad2b85e247 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0092000000-1244dc9085582bea8aeb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-04430922647f7b1ae254 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-0091000000-cadd5dca8babaae49f88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-9440000000-cbe7cd7dc7839e28d78b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0093000000-017912cf5f1aa782635c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0190000000-3cdf1772d9f3501b7e49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-3930000000-6d5acca31ee869a0bfd8 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|