| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:01:47 UTC |
|---|
| Update Date | 2020-04-22 18:56:17 UTC |
|---|
| BMDB ID | BMDB0096136 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
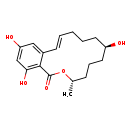
| Common Name | alpha-Zearalenol |
|---|
| Description | alpha-Zearalenol, also known as trans-zearalenol, belongs to the class of organic compounds known as macrolides and analogues. These are organic compounds containing a lactone ring of at least twelve members. Based on a literature review a small amount of articles have been published on alpha-Zearalenol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| trans-Zearalenol | Kegg | | a-Zearalenol | Generator | | Α-zearalenol | Generator | | alpha-Zearalenol, (cis)-isomer | HMDB | | Zearalenol | HMDB | | beta-Zearalenol | HMDB | | (3R,7R,11E)-7,14,16-Trihydroxy-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-1-one | HMDB | | beta-trans-Zearalenol | HMDB | | (-)-beta-Zearalenol | HMDB | | 3,4,5,6,7,8,9,10-Octahydro-7,14,16-trihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1-one | HMDB |
|
|---|
| Chemical Formula | C18H24O5 |
|---|
| Average Molecular Weight | 320.3802 |
|---|
| Monoisotopic Molecular Weight | 320.162373878 |
|---|
| IUPAC Name | (3S,7R)-7,14,16-trihydroxy-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-2-benzoxacyclotetradecin-1-one |
|---|
| Traditional Name | zeranol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@H]1CCC[C@H](O)CCC\C=C\C2=CC(O)=CC(O)=C2C(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C18H24O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,14,19-21H,2,4-6,8-9H2,1H3/b7-3+/t12-,14+/m0/s1 |
|---|
| InChI Key | FPQFYIAXQDXNOR-QDKLYSGJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macrolides and analogues. These are organic compounds containing a lactone ring of at least twelve members. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolides and analogues |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macrolides and analogues |
|---|
| Alternative Parents | |
|---|
| Substituents | - Macrolide
- Dihydroxybenzoic acid
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Vinylogous acid
- Carboxylic acid ester
- Lactone
- Secondary alcohol
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-0049000000-a87e9d1011a2491c8ddb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0229-8500950000-f4d3c9bdf8d5f08d6cad | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0019000000-4e38670336b2fa219440 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-5489000000-c9486dc02bd2970e1610 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0900-7920000000-b32caa0abd80ca28eabe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0019000000-62edd83447cda917e4cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-1119000000-9ed06dfe32fa5c630c0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-2390000000-eec44bc289fefe24387f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-116f24373daa24d943ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-65d407b0c8387d27535d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8091000000-fdac99a2354a0b46e9cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-ab0d3e9427f8c5216001 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0009000000-930d5de96dccc6bd3e83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-1092000000-12d134b2e63ea616dcd9 | View in MoNA |
|---|
|
|---|