| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:00:06 UTC |
|---|
| Update Date | 2020-04-22 18:55:38 UTC |
|---|
| BMDB ID | BMDB0096033 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
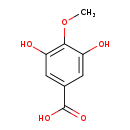
| Common Name | 4-O-Methylgallic acid |
|---|
| Description | 4-O-Methylgallic acid, also known as 4-O-methylgallate or 4OMGA CPD, belongs to the class of organic compounds known as gallic acid and derivatives. Gallic acid and derivatives are compounds containing a 3,4,5-trihydroxybenzoic acid moiety. Based on a literature review very few articles have been published on 4-O-Methylgallic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-O-Methylgallate | Generator | | 3,5-Dihydroxy-4-methoxybenzoic acid | MeSH | | 4-Methoxy-3,5-dihydroxybenzoic acid | MeSH | | 4-Methoxygallic acid | MeSH | | 4OMGA CPD | MeSH | | 4-Methoxygallate | Generator, HMDB | | 3,5-Dihydroxy-4-methoxy-benzoate | HMDB | | 3,5-Dihydroxy-4-methoxy-benzoic acid | HMDB | | 3,5-Dihydroxy-4-methoxybenzoate | HMDB, Generator | | 3,5-Dihydroxy-P-anisic acid | HMDB | | 4-Methoxy-3,5-dihydroxybenzoate | HMDB | | 5-Hydroxyisovanillic acid | HMDB | | 4-O-Methylgallic acid | MeSH |
|
|---|
| Chemical Formula | C8H8O5 |
|---|
| Average Molecular Weight | 184.1461 |
|---|
| Monoisotopic Molecular Weight | 184.037173366 |
|---|
| IUPAC Name | 3,5-dihydroxy-4-methoxybenzoic acid |
|---|
| Traditional Name | 3,5-dihydroxy-4-methoxybenzoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=C(O)C=C(C=C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C8H8O5/c1-13-7-5(9)2-4(8(11)12)3-6(7)10/h2-3,9-10H,1H3,(H,11,12) |
|---|
| InChI Key | UBXDWYFLYYJQFR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gallic acid and derivatives. Gallic acid and derivatives are compounds containing a 3,4,5-trihydroxybenzoic acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Gallic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gallic acid or derivatives
- P-methoxybenzoic acid or derivatives
- Methoxyphenol
- Benzoic acid
- Phenoxy compound
- Anisole
- Methoxybenzene
- Resorcinol
- Phenol ether
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0159-1900000000-32a93cf4f7568a9bdedc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-009i-2139000000-adf8b88e8bb85a43cb0f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-a129a3b3cfd0493a0408 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-4576c6e0e594b56d93b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-2900000000-19f8938cc76bae18f2e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-886cb3b61676c31904a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0080-0900000000-4bad45b0a4df1de7f271 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-2900000000-17d90b668a07c63b811e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-25175f7a5af29e8cfca5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0900000000-f2f0c47926cb84d06fdd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05n0-9000000000-eb7116993f3b670a52cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-962a559c43543c7fb0cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-0900000000-4cfc053bdff3ca1768bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9200000000-548c94ddc8cc64cba53b | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|