| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:59:36 UTC |
|---|
| Update Date | 2020-04-22 18:55:27 UTC |
|---|
| BMDB ID | BMDB0096002 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Noradrenochrome |
|---|
| Description | Noradrenochrome belongs to the class of organic compounds known as indoles and derivatives. These are organic compounds containing an indole, which is a bicyclic ring system made up of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Based on a literature review very few articles have been published on Noradrenochrome. |
|---|
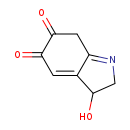
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,6-dioxo-2,3,5,6-tetrahydro-3-Hydroxyindole | HMDB | | 5,6-dioxo-2,3,5,6-tetrahydro-3-Indolol | HMDB | | AdChr | HMDB | | Noradrenochrome O-quinone | HMDB | | Noradrenochrome oxidised | HMDB | | Cyclized norepinephrine ortho-quinone | MeSH |
|
|---|
| Chemical Formula | C8H7NO3 |
|---|
| Average Molecular Weight | 165.1461 |
|---|
| Monoisotopic Molecular Weight | 165.042593095 |
|---|
| IUPAC Name | 3-hydroxy-3,5,6,7-tetrahydro-2H-indole-5,6-dione |
|---|
| Traditional Name | 3-hydroxy-3,7-dihydro-2H-indole-5,6-dione |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC1CN=C2CC(=O)C(=O)C=C12 |
|---|
| InChI Identifier | InChI=1S/C8H7NO3/c10-6-1-4-5(2-7(6)11)9-3-8(4)12/h1,8,12H,2-3H2 |
|---|
| InChI Key | PTQNKGWWIYGAAE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indoles and derivatives. These are organic compounds containing an indole, which is a bicyclic ring system made up of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Indoles and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indole or derivatives
- Cyclohexenone
- Pyrroline
- Ketimine
- Ketone
- Secondary alcohol
- Cyclic ketone
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Imine
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fs-8900000000-b8bf972fa7b384c177cb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-9640000000-94e5729c119b4f33b4fa | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-54a88fc5ae849b3139fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0900000000-02b253c9f61c98665071 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9400000000-78972a8172f41ceb21b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-f11f49deb557d5d11503 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-ac348f2387aeffb9e929 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-4900000000-1ff5a9799f5463f14220 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-65490e650f013cfe115e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-b87e3a72f8a872d73e25 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ac3-2900000000-5912cbe5d2d9cc0a8324 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-9505c7726be7ea1b51d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-d1deaf55837d69fc7853 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9500000000-d0f6a26748f4650d9c0d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|