| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:59:03 UTC |
|---|
| Update Date | 2020-04-22 18:55:15 UTC |
|---|
| BMDB ID | BMDB0095971 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
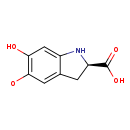
| Common Name | Dopachrome o-semiquinone |
|---|
| Description | Dopachrome o-semiquinone belongs to the class of organic compounds known as indolecarboxylic acids. Indolecarboxylic acids are compounds containing a carboxylic acid group linked to an indole. Based on a literature review very few articles have been published on Dopachrome o-semiquinone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| DCSQ | HMDB | | Leukodopachrome O-semiquinone radical | HMDB |
|
|---|
| Chemical Formula | C9H8NO4 |
|---|
| Average Molecular Weight | 194.1641 |
|---|
| Monoisotopic Molecular Weight | 194.045332749 |
|---|
| IUPAC Name | [(2R)-2-carboxy-6-hydroxy-2,3-dihydro-1H-indol-5-yl]oxidanyl |
|---|
| Traditional Name | (2R)-2-carboxy-6-hydroxy-2,3-dihydro-1H-indol-5-yloxidanyl |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [O]C1=C(O)C=C2N[C@H](CC2=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H8NO4/c11-7-2-4-1-6(9(13)14)10-5(4)3-8(7)12/h2-3,6,10,12H,1H2,(H,13,14)/t6-/m1/s1 |
|---|
| InChI Key | YHAPBGUGQAHLME-ZCFIWIBFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolecarboxylic acids. Indolecarboxylic acids are compounds containing a carboxylic acid group linked to an indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolecarboxylic acids and derivatives |
|---|
| Direct Parent | Indolecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolecarboxylic acid
- Alpha-amino acid
- Alpha-amino acid or derivatives
- D-alpha-amino acid
- Dihydroindole
- 1-hydroxy-2-unsubstituted benzenoid
- Secondary aliphatic/aromatic amine
- Aralkylamine
- Benzenoid
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fk9-0900000000-14161f2f212d7f618988 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00r6-9862000000-48bde12fcf4b33d59d79 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0900000000-b20012b2274681d12add | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0900000000-c837b0952c9118b19617 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-3900000000-c08c219ad8015ee050bd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-d95cfc7e3a54a6f9a4a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-21cbd7b3c97ef0183c57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-4900000000-c16a28629a2b109bcf85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-17f8e7f25df3a1f125cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-436b7bee27cb61484cd8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dj-1900000000-a8a00903984a194ba494 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|