| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:52 UTC |
|---|
| Update Date | 2020-04-22 18:55:10 UTC |
|---|
| BMDB ID | BMDB0095959 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
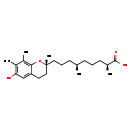
| Common Name | 9'-Carboxy-gamma-chromanol |
|---|
| Description | 9'-Carboxy-gamma-chromanol, also known as γ-cdmohc or gamma-cdmohc, belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. Based on a literature review very few articles have been published on 9'-Carboxy-gamma-chromanol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,6R)-9-[(2R)-6-Hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-2,6-dimethylnonanoate | HMDB | | Γ-cdmohc | HMDB | | gamma-CDMOHC | HMDB | | γ-Carboxydimethyloctyl hydroxychroman | HMDB | | γ-Carboxydimethyloctylhydroxychroman | HMDB | | gamma-Carboxydimethyloctyl hydroxychroman | HMDB | | gamma-Carboxydimethyloctylhydroxychroman | HMDB |
|
|---|
| Chemical Formula | C23H36O4 |
|---|
| Average Molecular Weight | 376.537 |
|---|
| Monoisotopic Molecular Weight | 376.261359639 |
|---|
| IUPAC Name | (2S,6R)-9-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-2,6-dimethylnonanoic acid |
|---|
| Traditional Name | (2S,6R)-9-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-1-benzopyran-2-yl]-2,6-dimethylnonanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@H](CCC[C@H](C)C(O)=O)CCC[C@]1(C)CCC2=CC(O)=C(C)C(C)=C2O1 |
|---|
| InChI Identifier | InChI=1S/C23H36O4/c1-15(8-6-10-16(2)22(25)26)9-7-12-23(5)13-11-19-14-20(24)17(3)18(4)21(19)27-23/h14-16,24H,6-13H2,1-5H3,(H,25,26)/t15-,16+,23-/m1/s1 |
|---|
| InChI Key | ODQOKHVJIYTOQE-VBURHUQHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-benzopyran
- Medium-chain fatty acid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kdi-0419000000-cd057edae393743570b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0911000000-a634d5d40e9ad8466db6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-2900000000-567171c8ebdc774c19a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-8be58d31ef5f29daf85c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-005a-0409000000-c4f9d5fd1cfb91f60b96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05tb-7916000000-27664edfcab573c04ee2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ufr-1259000000-07af3dd22eab013841cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0012-1492000000-34cf07d467d81a9bf6a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fe3-4920000000-f1c204588a76056667ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-8cfda78ea5a5ec24a352 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0509000000-c1a7322cafcde235713d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02ou-1924000000-878d6d96351e5c61af97 | View in MoNA |
|---|
|
|---|