| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:51 UTC |
|---|
| Update Date | 2020-04-22 18:55:10 UTC |
|---|
| BMDB ID | BMDB0095958 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 9'-Carboxy-alpha-tocotrienol |
|---|
| Description | 9'-Carboxy-alpha-tocotrienol belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. Based on a literature review a small amount of articles have been published on 9'-Carboxy-alpha-tocotrienol. |
|---|
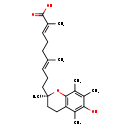
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 9'-Carboxy-a-tocotrienol | Generator | | 9'-Carboxy-α-tocotrienol | Generator | | alpha-CDMOenHC | HMDB | | (2E,6E)-9-[(2R)-6-Hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-2,6-dimethylnona-2,6-dienoate | Generator |
|
|---|
| Chemical Formula | C24H34O4 |
|---|
| Average Molecular Weight | 386.5244 |
|---|
| Monoisotopic Molecular Weight | 386.245709576 |
|---|
| IUPAC Name | (2E,6E)-9-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-2,6-dimethylnona-2,6-dienoic acid |
|---|
| Traditional Name | (2E,6E)-9-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-1-benzopyran-2-yl]-2,6-dimethylnona-2,6-dienoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C\C(CC\C=C(/C)C(O)=O)=C/CC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2C |
|---|
| InChI Identifier | InChI=1S/C24H34O4/c1-15(9-7-11-16(2)23(26)27)10-8-13-24(6)14-12-20-19(5)21(25)17(3)18(4)22(20)28-24/h10-11,25H,7-9,12-14H2,1-6H3,(H,26,27)/b15-10+,16-11+/t24-/m1/s1 |
|---|
| InChI Key | MEZYOZBFEXYIKB-ASKDMKCJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Bicyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aromatic monoterpenoid
- Chromane
- Benzopyran
- Bicyclic monoterpenoid
- 1-benzopyran
- Medium-chain fatty acid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Unsaturated fatty acid
- Organoheterocyclic compound
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05g3-3597000000-ee33a4040e8ff3c5f3a2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-2165490000-8847e41fbbef01f7bda5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0429000000-a66826ff0d07b0106393 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0912000000-5ee1236a995a9d12f0d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-1910000000-ee9f7f94c621608c3fb3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-b373da74391ce2789a05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03du-0319000000-17f654138f67f0a81c49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02cs-1923000000-50e6ff8d4a1ad04e4c79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-0009000000-bee69c7476b7b7fa661c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0109000000-24ce754465533875736b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0lmi-0192000000-3f4ee4b78e71bf13cccf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0300-0097000000-c797498613a30824a81a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066v-0092000000-84aa2ba2bc2336336b65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5980000000-d16bab22c4bb256a7101 | View in MoNA |
|---|
|
|---|