| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:48 UTC |
|---|
| Update Date | 2020-04-22 18:55:09 UTC |
|---|
| BMDB ID | BMDB0095955 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7'-Carboxy-gamma-chromanol |
|---|
| Description | 7'-Carboxy-gamma-chromanol, also known as Γ-CMHHC or gamma-CMHHC, belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. Based on a literature review very few articles have been published on 7'-Carboxy-gamma-chromanol. |
|---|
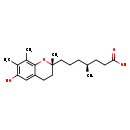
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4S)-7-[(2R)-6-Hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylheptanoate | HMDB | | Γ-CMHHC | HMDB | | gamma-CMHHC | HMDB | | γ-Carboxymethylhexyl hydroxychroman | HMDB | | γ-Carboxymethylhexylhydroxychroman | HMDB | | gamma-Carboxymethylhexyl hydroxychroman | HMDB | | gamma-Carboxymethylhexylhydroxychroman | HMDB |
|
|---|
| Chemical Formula | C20H30O4 |
|---|
| Average Molecular Weight | 334.456 |
|---|
| Monoisotopic Molecular Weight | 334.214409446 |
|---|
| IUPAC Name | (4S)-7-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylheptanoic acid |
|---|
| Traditional Name | (4S)-7-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-1-benzopyran-2-yl]-4-methylheptanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@@H](CCC[C@]1(C)CCC2=CC(O)=C(C)C(C)=C2O1)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H30O4/c1-13(7-8-18(22)23)6-5-10-20(4)11-9-16-12-17(21)14(2)15(3)19(16)24-20/h12-13,21H,5-11H2,1-4H3,(H,22,23)/t13-,20+/m0/s1 |
|---|
| InChI Key | JFXDIXKMINYLIK-RNODOKPDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-benzopyran
- Medium-chain fatty acid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0539000000-6050268e609a16466634 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1921000000-7fda212da468ee981e95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1000-5900000000-5c8150efaf5eae1bce1f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0119000000-9efcbf3debf6e3fab178 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lb-1749000000-0ef6f3c235f3a19bf981 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-9841000000-7e52b90564e14a9bfb9e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00li-0059000000-951a5f97c523abed2dbc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-2936000000-ad8508fe5d137c7c62b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0076-2951000000-edc264cfd7d7cc48b18b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00ks-0195000000-f475a6e7b1f959cd7904 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1390000000-abe1846fbee337e380b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdo-5900000000-892525c38c02793675e1 | View in MoNA |
|---|
|
|---|