| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-03 19:35:50 UTC |
|---|
| Update Date | 2020-04-22 15:56:33 UTC |
|---|
| BMDB ID | BMDB0064015 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methionyl-Gamma-glutamate |
|---|
| Description | Methionyl-Gamma-glutamate, also known as m-ge dipeptide or methionyl-g-glutamic acid, belongs to the class of organic compounds known as glutamine and derivatives. Glutamine and derivatives are compounds containing glutamine or a derivative thereof resulting from reaction of glutamine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review very few articles have been published on Methionyl-Gamma-glutamate. |
|---|
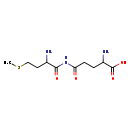
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methionyl-g-glutamate | Generator | | Methionyl-g-glutamic acid | Generator | | Methionyl-gamma-glutamic acid | Generator | | Methionyl-γ-glutamate | Generator | | Methionyl-γ-glutamic acid | Generator | | L-Methionyl-L-gamma-glutamate | HMDB | | m-GE dipeptide | HMDB | | Met-gglu | HMDB | | Methionine gamma-glutamate dipeptide | HMDB | | Methionine-gamma-glutamate dipeptide | HMDB | | Methionylgamma-glutamate | HMDB | | MGE dipeptide | HMDB | | 2-Amino-4-{[2-amino-4-(methylsulfanyl)butanoyl]-C-hydroxycarbonimidoyl}butanoate | HMDB | | 2-Amino-4-{[2-amino-4-(methylsulphanyl)butanoyl]-C-hydroxycarbonimidoyl}butanoate | HMDB | | 2-Amino-4-{[2-amino-4-(methylsulphanyl)butanoyl]-C-hydroxycarbonimidoyl}butanoic acid | HMDB |
|
|---|
| Chemical Formula | C10H19N3O4S |
|---|
| Average Molecular Weight | 277.341 |
|---|
| Monoisotopic Molecular Weight | 277.109626801 |
|---|
| IUPAC Name | 2-amino-4-{[2-amino-4-(methylsulfanyl)butanoyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | 2-amino-4-{[2-amino-4-(methylsulfanyl)butanoyl]carbamoyl}butanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CSCCC(N)C(=O)NC(=O)CCC(N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H19N3O4S/c1-18-5-4-6(11)9(15)13-8(14)3-2-7(12)10(16)17/h6-7H,2-5,11-12H2,1H3,(H,16,17)(H,13,14,15) |
|---|
| InChI Key | YVSZXQHOADMOLO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamine and derivatives. Glutamine and derivatives are compounds containing glutamine or a derivative thereof resulting from reaction of glutamine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamine or derivatives
- Methionine or derivatives
- Alpha-amino acid amide
- Alpha-amino acid
- Fatty acid
- N-acyl-amine
- Carboxylic acid imide
- Dicarboximide
- Carboxylic acid imide, n-unsubstituted
- Amino acid
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Amine
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zgi-9630000000-6f8b1b8a730c9ab9cf14 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0un9-9521000000-2e5e40c81ac8ea0d2709 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-1970000000-dc6a6120bb5558357ec7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-3910000000-d0db9ede142f61abf380 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc0-9200000000-885319e7abcab6982f8e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004j-5190000000-3fcc7169af70b32481d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9320000000-2bbfe62e559d48ad44f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9200000000-631b7aee82c96f11704e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0490000000-6097f2c2adeb9b663091 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01u0-0490000000-c9aa17f0fc2718af5c6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1159-9600000000-00034b5d57eb4e8d974a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-f6e6d4487d4a782fb615 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9820000000-595f17019036e20abd42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-9100000000-51e4312361bd4fe1e0a1 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|