| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-03 19:24:26 UTC |
|---|
| Update Date | 2020-04-22 15:55:19 UTC |
|---|
| BMDB ID | BMDB0063819 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Aspartyl-Lysine |
|---|
| Description | Aspartyl-Lysine, also known as D-K dipeptide or asp-lys, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Based on a literature review very few articles have been published on Aspartyl-Lysine. |
|---|
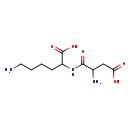
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Asp-lys | HMDB | | Aspartate lysine dipeptide | HMDB | | Aspartate-lysine dipeptide | HMDB | | Aspartyllysine | HMDB | | D-K Dipeptide | HMDB | | DK Dipeptide | HMDB | | L-Aspartyl-L-lysine | HMDB | | 6-Amino-2-[(2-amino-3-carboxy-1-hydroxypropylidene)amino]hexanoate | HMDB | | N-epsilon-(beta-Aspartyl)lysine | HMDB | | beta-Aspartyl-epsilon-lysine | HMDB |
|
|---|
| Chemical Formula | C10H19N3O5 |
|---|
| Average Molecular Weight | 261.275 |
|---|
| Monoisotopic Molecular Weight | 261.132470733 |
|---|
| IUPAC Name | 6-amino-2-(2-amino-3-carboxypropanamido)hexanoic acid |
|---|
| Traditional Name | 6-amino-2-(2-amino-3-carboxypropanamido)hexanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NCCCCC(NC(=O)C(N)CC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H19N3O5/c11-4-2-1-3-7(10(17)18)13-9(16)6(12)5-8(14)15/h6-7H,1-5,11-12H2,(H,13,16)(H,14,15)(H,17,18) |
|---|
| InChI Key | OAMLVOVXNKILLQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Aspartic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Medium-chain fatty acid
- Amino fatty acid
- N-acyl-amine
- Fatty amide
- Dicarboxylic acid or derivatives
- Fatty acid
- Fatty acyl
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Amino acid
- Carboxylic acid
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-9110000000-8b8dc801768525722b39 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03di-4921000000-f012988a061095d9862a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002g-1290000000-2dec0edff4c986109982 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-9750000000-91f545265e3caa58f1cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-da0ac6e5b33e2e45c27e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0290000000-5685db7f9c81e4950e45 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0295-1960000000-2a3bcd0c8b05bda15140 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-8900000000-52194486dfd805dfcd05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0390000000-2a23f7f9e14b26c56a52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-7940000000-b5a58a78f3b1ba72a5fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-9300000000-87559a28c6b8de194e95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-b1ac2aa01050399fdeb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2920000000-9ef037efadfdb8fa2c84 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-57aff7f2ce790f6800ce | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|