| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-11-08 19:40:12 UTC |
|---|
| Update Date | 2020-06-04 23:01:27 UTC |
|---|
| BMDB ID | BMDB0063634 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Simazine |
|---|
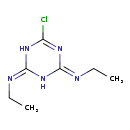
| Description | Simazine, also known as gesatop or princep, belongs to the class of organic compounds known as chloro-s-triazines. These are aromatic compounds containing a 1,3,5-triazine ring that is substituted at the 2-position with a chlorine atom. Based on a literature review a significant number of articles have been published on Simazine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4-Bis(ethylamino)-6-chloro-1,3,5-triazine | ChEBI | | 2,4-Bis(ethylamino)-6-chloro-S-triazine | ChEBI | | 2-Chloro-4,6-bis(ethylamino)-1,3,5-triazine | ChEBI | | 2-Chloro-4,6-bis(ethylamino)-S-triazine | ChEBI | | 6-Chloro-N,n'-diethyl-[1,3,5]triazin-2,4-diamine | ChEBI | | 6-Chloro-N(2),N(4)-diethyl-1,3,5-triazine-2,4-diamine | ChEBI | | Gesatop | ChEBI | | Princep | ChEBI | | Simanex | ChEBI | | Herbazin-50 | MeSH | | Herbazin 50 | MeSH | | Herbazin50 | MeSH |
|
|---|
| Chemical Formula | C7H12ClN5 |
|---|
| Average Molecular Weight | 201.657 |
|---|
| Monoisotopic Molecular Weight | 201.078123116 |
|---|
| IUPAC Name | N-[6-chloro-4-(ethylimino)-1,2,3,4-tetrahydro-1,3,5-triazin-2-ylidene]ethan-1-amine |
|---|
| Traditional Name | N-[4-chloro-6-(ethylimino)-1,3-dihydro-1,3,5-triazin-2-ylidene]ethanamine |
|---|
| CAS Registry Number | 122-34-9 |
|---|
| SMILES | CCN=C1NC(Cl)=NC(N1)=NCC |
|---|
| InChI Identifier | InChI=1S/C7H12ClN5/c1-3-9-6-11-5(8)12-7(13-6)10-4-2/h3-4H2,1-2H3,(H2,9,10,11,12,13) |
|---|
| InChI Key | ODCWYMIRDDJXKW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chloro-s-triazines. These are aromatic compounds containing a 1,3,5-triazine ring that is substituted at the 2-position with a chlorine atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazines |
|---|
| Sub Class | 1,3,5-triazines |
|---|
| Direct Parent | Chloro-s-triazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chloro-s-triazine
- Aryl halide
- Aryl chloride
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00b9-6910000000-6f6b1db95e5cccd40b3f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0ul0-2940000000-30748c0e2f5b82122f7f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0udi-0090000000-3e6023b6250decbed346 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0fk9-0930000000-8466abb0e5695be2230c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0fk9-0940000000-494887c450be4e0bfdeb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0ul0-3930000000-c0c85fd651a37dad8931 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0gb9-9300000000-0b08d00c2d7364671fff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0v4j-9600000000-57b2139a668014996ab0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 55V, Positive | splash10-00di-0900000000-9919aee0cbe7d1711f5f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0002-0900000000-6bf03adbaeef3e6ff012 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0udi-0590000000-e8d76becfcf4abb9b434 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0udi-0090000000-e04c3b23b2c44d0cd2e9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 80V, Positive | splash10-0udi-4900000000-36e642f3a70c9c883515 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0fl0-0910000000-cbb03ebba1105d912842 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0ul0-3920000000-c6dbff95726e49eb75a0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0900000000-26f2121606fb90189a41 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0002-0920000000-0c6930de734cdd1d492a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0590000000-554571e4ddb17bb31bc0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0v5a-7900000000-3e5ae9cb5c0f037f4a14 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0udi-0590000000-06c08d64ab959b2d694f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0290000000-7af8d2aec614ed62e696 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-2950000000-2e6e03117a62e4f44059 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-eafdd57a078143a4732f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f79-2930000000-13f0420da11a492b7f7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ukc-9840000000-51df93e4d6d15aa43ab6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8900000000-cd303711ba1aab711ad2 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0udu-9620000000-8505155b2e594b1bb091 | View in MoNA |
|---|
|
|---|