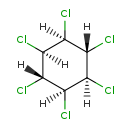
| Description | Lindane, also known as gamma-hexachlorocyclohexane (γ-HCH), gammaxene, Gammallin is an organochlorine insecticide that has been used as a pediculicide and a scabicide. Lindane has been banned in California, United Kingdom, Australia, and many western countries due to concerns about neurotoxicity and adverse effects on the environment. In Canada, Lindane is not recommmended as a first-line therapy due to reports of resistance, neurotoxicity, and bone marrow suppression, but has been approved by the FDA as a second-line therapy for topical treatment of pediculosis capitis (head lice), pediculosis pubis (pubic lice), or scabies in patients greater than two years of age who cannot tolerate or have failed first-line treatment. |
|---|
| Synonyms | | Value | Source |
|---|
| (1alpha,2alpha,3beta,4alpha,5alpha,6beta)-1,2,3,4,5,6-Hexachlorocyclohexane | ChEBI | | (1R,2C,3t,4C,5C,6t)-1,2,3,4,5,6-Hexachlorocyclohexane | ChEBI | | 1,2,3,4,5,6-Hexachlorocyclohexane | ChEBI | | Benzene hexachloride | ChEBI | | gamma-1,2,3,4,5,6-Hexachlorocyclohexane | ChEBI | | gamma-Benzene hexachloride | ChEBI | | gamma-BHC | ChEBI | | gamma-HCH | ChEBI | | gamma-Hexachlorzyklohexan | ChEBI | | gamma-Lindane | ChEBI | | Kwell | ChEBI | | Lindan | ChEBI | | gamma-Hexachlorocyclohexane | Kegg | | (1a,2a,3b,4a,5a,6b)-1,2,3,4,5,6-Hexachlorocyclohexane | Generator | | (1Α,2α,3β,4α,5α,6β)-1,2,3,4,5,6-hexachlorocyclohexane | Generator | | g-1,2,3,4,5,6-Hexachlorocyclohexane | Generator | | Γ-1,2,3,4,5,6-hexachlorocyclohexane | Generator | | g-Benzene hexachloride | Generator | | Γ-benzene hexachloride | Generator | | g-BHC | Generator | | Γ-BHC | Generator | | g-HCH | Generator | | Γ-HCH | Generator | | g-Hexachlorzyklohexan | Generator | | Γ-hexachlorzyklohexan | Generator | | g-Lindane | Generator | | Γ-lindane | Generator | | g-Hexachlorocyclohexane | Generator | | Γ-hexachlorocyclohexane | Generator | | Lindane | ChEBI |
|
|---|