| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:19:43 UTC |
|---|
| Update Date | 2020-05-11 20:42:55 UTC |
|---|
| BMDB ID | BMDB0062213 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methylcysteine |
|---|
| Description | S-Methylcysteine belongs to the class of organic compounds known as l-cysteine-s-conjugates. L-cysteine-S-conjugates are compounds containing L-cysteine where the thio-group is conjugated. Based on a literature review a significant number of articles have been published on S-Methylcysteine. |
|---|
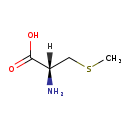
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2R)-2-Amino-3-(methylsulfanyl)propanoic acid | ChEBI | | (R)-2-Amino-3-(methylthio)propanoic acid | ChEBI | | 3-(Methylthio)-L-alanine | ChEBI | | L-Methylcysteine | ChEBI | | S-Methyl-L-cysteine | ChEBI | | (2R)-2-Amino-3-(methylsulfanyl)propanoate | Generator | | (2R)-2-Amino-3-(methylsulphanyl)propanoate | Generator | | (2R)-2-Amino-3-(methylsulphanyl)propanoic acid | Generator | | (R)-2-Amino-3-(methylthio)propanoate | Generator | | 3-(methylthio)-L-(8CI)alanine | HMDB | | Acm-thiopropionate | HMDB | | Acm-thiopropionic acid | HMDB | | L-Aspartic acid dimethyl ester | HMDB | | S-Acetamidomethyl-deamino-cysteine | HMDB | | S-Methyl-(9ci)-L-cysteine | HMDB | | S-Methyl-cysteine | HMDB | | S-Methyl-DL-cysteine | HMDB | | S-Methylcysteine | HMDB, MeSH | | S-11C-Methyl-L-cysteine | MeSH, HMDB | | S-Methylcysteine, (DL-cys)-isomer | MeSH, HMDB | | S-Methylcysteine, hydrochloride, (L-cys)-isomer | MeSH, HMDB | | S-Methylcysteine, (L-cys)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C4H9NO2S |
|---|
| Average Molecular Weight | 135.185 |
|---|
| Monoisotopic Molecular Weight | 135.035399227 |
|---|
| IUPAC Name | (2R)-2-amino-3-(methylsulfanyl)propanoic acid |
|---|
| Traditional Name | S-methylcysteine |
|---|
| CAS Registry Number | 1187-84-4 |
|---|
| SMILES | [H][C@](N)(CSC)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1 |
|---|
| InChI Key | IDIDJDIHTAOVLG-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-cysteine-s-conjugates. L-cysteine-S-conjugates are compounds containing L-cysteine where the thio-group is conjugated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-cysteine-S-conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-cysteine-s-conjugate
- Alpha-amino acid
- L-alpha-amino acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Thioether
- Sulfenyl compound
- Dialkylthioether
- Carbonyl group
- Amine
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-0006-9500000000-daa44ef1254b2abffd37 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-02t9-1950000000-7418765e9cc42b0155e4 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fv-9000000000-d790345dc4143a2c43ef | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0006-9500000000-daa44ef1254b2abffd37 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-02t9-1950000000-7418765e9cc42b0155e4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-72d010b2583bd53b7e08 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006y-9400000000-3b5fc90a341033d6e213 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-1900000000-4a373aa1d088e914bead | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00b9-9000000000-04c165848579841841db | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00fr-9000000000-1b77045ef8e2cb3b10e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-03fv-9000000000-9377aea80fe674b0a8de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-9700000000-8f3d25ac52279c092856 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9200000000-b405850a71df669cec12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-9000000000-ace8060e79e2568e8fd6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9200000000-0100ccbfcee39a28f73d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000b-9000000000-77f7473e8f64b00cba29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9000000000-5f83877d00d54983d86c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01bc-7900000000-15e4c05adb56715c93d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dm-9000000000-5a8e96324e324aab8703 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-c69e8c7f456c0f6fa275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-e1d92d2a30bee517d754 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9000000000-e1d92d2a30bee517d754 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9000000000-e1d92d2a30bee517d754 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|