| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-06-25 21:46:25 UTC |
|---|
| Update Date | 2020-04-22 15:51:13 UTC |
|---|
| BMDB ID | BMDB0062045 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
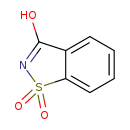
| Common Name | Saccharin |
|---|
| Description | Saccharin, also known as benzosulfimide or O-sulfobenzimide, belongs to the class of organic compounds known as benzothiazoles. These are organic compounds containing a benzene fused to a thiazole ring (a five-membered ring with four carbon atoms, one nitrogen atom and one sulfur atom). Saccharin exists as a solid, possibly soluble (in water), and a moderately acidic compound (based on its pKa) molecule. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,1-Dioxo-1,2-benzisothiazol-3(2H)-one | ChEBI | | 1,1-Dioxo-1,2-dihydro-benzo[D]isothiazol-3-one | ChEBI | | 1,2-Benzisothiazol-3(2H)-one 1,1-dioxide | ChEBI | | 1,2-Benzisothiazolin-3-one 1,1-dioxide | ChEBI | | 1,2-Dihydro-2-ketobenzisosulfonazole | ChEBI | | 1,2-Dihydro-2-ketobenzisosulphonazole | ChEBI | | 2,3-Dihydro-3-oxobenzisosulfonazole | ChEBI | | 2,3-Dihydro-3-oxobenzisosulphonazole | ChEBI | | 3-Hydroxybenzisothiazole-S,S-dioxide | ChEBI | | Anhydro-O-sulfaminebenzoic acid | ChEBI | | Benzo-2-sulphimide | ChEBI | | Benzoic acid sulfimide | ChEBI | | Benzoic sulfimide | ChEBI | | Benzoic sulphimide | ChEBI | | Benzosulfimide | ChEBI | | Benzosulphimide | ChEBI | | Benzoylsulfonic imide | ChEBI | | O-Benzoic sulfimide | ChEBI | | O-Benzosulfimide | ChEBI | | O-Sulfobenzimide | ChEBI | | O-Sulfobenzoic acid imide | ChEBI | | Saccharimide | ChEBI | | Saccharine | ChEBI | | Sweeta | Kegg | | Anhydro-O-sulfaminebenzoate | Generator | | Anhydro-O-sulphaminebenzoate | Generator | | Anhydro-O-sulphaminebenzoic acid | Generator | | Benzo-2-sulfimide | Generator | | Benzoate sulfimide | Generator | | Benzoate sulphimide | Generator | | Benzoic acid sulphimide | Generator | | Benzoylsulphonic imide | Generator | | O-Benzoic sulphimide | Generator | | O-Benzosulphimide | Generator | | O-Sulphobenzimide | Generator | | O-Sulfobenzoate imide | Generator | | O-Sulphobenzoate imide | Generator | | O-Sulphobenzoic acid imide | Generator | | 1, 2-Benzisothiazol-3(2H)-one, 1,1-dioxide | HMDB | | 1, 2-Benzisothiazolin-3-one 1,1-dioxide | HMDB | | 1, 2-dihydro-2-Ketobenzisosulfonazole | HMDB | | 1,1-Diox-1,2-benzisothiazol-3-one | HMDB | | 1,1-Dioxide-1,2-benzisothiazol-3(2H)-one | HMDB | | 1,1-Dioxide-1,2-benzisothiazolin-3-one | HMDB | | 1,1-dioxo-1,2-dihydro-1Lambda*6*-benzo[D]isothiazol-3-one | HMDB | | 1,2-Benzisothiazol-3(2H)-one 1,1-dioxide, 9ci | HMDB | | 1,2-Benzisothiazol-3(2H)-one, 1,1-dioxide | HMDB | | 1,2-Benzisothiazolin-3-one, 1,1-dioxide | HMDB | | 1,2-Benzisothiazoline-3-one 1,1-dioxide | HMDB | | 1,2-Benzothiazol-3(2H)-one 1,1-dioxide | HMDB | | 2, 3-dihydro-3-Oxobenzisosulfonazole | HMDB | | 2,3-dihydro-1,2-Benzoisothiazol-3-one-1,1-dioxide | HMDB | | 2,3-dihydro-3-oxo-Benzisosulfonazole | HMDB | | 2,3-Dihydroxy-1,2-benzisothiazol-3-one-1,1-dioxide | HMDB | | 2-Sulfobenzoic acid imide | HMDB | | 2-Sulfobenzoicimide | HMDB | | 2-Sulphobenzoic imide | HMDB | | 3-Benzisothiazolinone 1, 1-dioxide | HMDB | | 3-Benzisothiazolinone 1,1-dioxide | HMDB | | 3-Hydroxybenzisothiazole S,S-dioxide | HMDB | | benzo-2-Sulfiide | HMDB | | benzo-Sulphinide | HMDB | | Benzosulfinide | HMDB | | e954 | HMDB | | Garantose | HMDB | | Glucid | HMDB | | Gluside | HMDB | | Glycophenol | HMDB | | Hermesetas | HMDB | | Insoluble saccharin | HMDB | | Kandiset | HMDB | | LSA | HMDB | | Neosaccharin | HMDB | | O-Benzoic acid sulfimide | HMDB | | O-Benzoyl sulfimide | HMDB | | O-Benzoyl sulphimide | HMDB | | O-Benzoylsulfimide | HMDB | | O-Sulfobenzoic imide | HMDB | | Sacarina | HMDB | | Saccharin (JP15/nf) | HMDB | | Saccharin acid | HMDB | | Saccharin insoluble | HMDB | | Saccharin, insoluble | HMDB | | Saccharinol | HMDB | | Saccharinose | HMDB | | Saccharol | HMDB | | Sacharin | HMDB | | Stilalgin | HMDB | | Sucre edulcor | HMDB | | Sucrette | HMDB | | Syncal | HMDB | | Zaharina | HMDB | | Saccharin sodium | MeSH, HMDB | | Saccharin calcium | MeSH, HMDB | | Calcium, saccharin | MeSH, HMDB |
|
|---|
| Chemical Formula | C7H5NO3S |
|---|
| Average Molecular Weight | 183.185 |
|---|
| Monoisotopic Molecular Weight | 182.999013721 |
|---|
| IUPAC Name | 2,3-dihydro-1lambda6,2-benzothiazole-1,1,3-trione |
|---|
| Traditional Name | 2H-1lambda6,2-benzothiazole-1,1,3-trione |
|---|
| CAS Registry Number | 81-07-2 |
|---|
| SMILES | OC1=NS(=O)(=O)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C7H5NO3S/c9-7-5-3-1-2-4-6(5)12(10,11)8-7/h1-4H,(H,8,9) |
|---|
| InChI Key | CVHZOJJKTDOEJC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzothiazoles. These are organic compounds containing a benzene fused to a thiazole ring (a five-membered ring with four carbon atoms, one nitrogen atom and one sulfur atom). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazoles |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzothiazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-benzothiazole
- Benzenoid
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Azacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-006x-5940000000-c3d4fc7537245087aa01 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0fiv-9200000000-3cbd853d90d67ac630ab | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-006x-5940000000-c3d4fc7537245087aa01 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0006-2940000000-42addba0b789ed51670d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f89-2900000000-becdd7f75934373f608e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-4b6ed2bc790c007ba1d2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-1548a4ac4e5c19442679 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-34f1a9ce4b083851e69f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-20951911ba1a4f08d891 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-668e33ca497d39237e85 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-fdb874ff651646e63cab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-65c93c049826341beddd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-1548a4ac4e5c19442679 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-1548a4ac4e5c19442679 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-54eafaf51ea31a4d23c6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-d248f91c063ab27c21d0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-c92646389218a59f37b9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-1900000000-b50ceac4dba57f7be6c7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-73352f91026a3f00a604 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , negative | splash10-001i-0900000000-c9c9334260025c49503c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-001i-0900000000-1548a4ac4e5c19442679 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-8541df3050a01079f8cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-001l-7900000000-b92b2b5563f0682cf4c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-001i-0900000000-d11c013b29981671dfd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-a62962f93c65565c0ef0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-0900000000-9388e78a777b8d51a622 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2900000000-8b225ba6c3775d43faa3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-d247850c8363d99c278a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1900000000-6caddc90acdcf108de82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-db3e2a46b225fde95a28 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|