| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:34:10 UTC |
|---|
| Update Date | 2020-04-22 15:49:02 UTC |
|---|
| BMDB ID | BMDB0012208 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
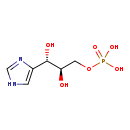
| Common Name | D-Erythro-imidazole-glycerol-phosphate |
|---|
| Description | D-Erythro-imidazole-glycerol-phosphate belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. D-Erythro-imidazole-glycerol-phosphate exists in all living species, ranging from bacteria to plants to humans. Based on a literature review very few articles have been published on D-Erythro-imidazole-glycerol-phosphate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Erythro-imidazole-glycerol 3-phosphate | ChEBI | | D-Erythro-imidazole-glycerol phosphate | ChEBI | | D-Erythro-imidazole-glycerol 3-phosphoric acid | Generator | | D-Erythro-imidazole-glycerol phosphoric acid | Generator | | D-Erythro-imidazole-glycerol-phosphoric acid | Generator | | D-erythro-1-(Imidazol-4-yl)glycerol 3-phosphate | HMDB | | D-erythro-Imidazoleglycerol-phosphate | HMDB | | D-erythro-Imidazole-glycerol-P | HMDB | | D-erythro-Imidazole-glycerol-phosphate | HMDB | | D-erythro-Imidazolylglycerol phosphate | HMDB | | IGP | HMDB | | Imidazole glycerol phosphate | HMDB | | erythro-Imidazole-glycerol-P | HMDB | | erythro-Imidazole-glycerol-phosphate | HMDB |
|
|---|
| Chemical Formula | C6H11N2O6P |
|---|
| Average Molecular Weight | 238.1351 |
|---|
| Monoisotopic Molecular Weight | 238.035472606 |
|---|
| IUPAC Name | [(2R,3S)-2,3-dihydroxy-3-(1H-imidazol-4-yl)propoxy]phosphonic acid |
|---|
| Traditional Name | (2R,3S)-2,3-dihydroxy-3-(1H-imidazol-4-yl)propoxyphosphonic acid |
|---|
| CAS Registry Number | 36244-87-8 |
|---|
| SMILES | O[C@H](COP(O)(O)=O)[C@@H](O)C1=CNC=N1 |
|---|
| InChI Identifier | InChI=1S/C6H11N2O6P/c9-5(2-14-15(11,12)13)6(10)4-1-7-3-8-4/h1,3,5-6,9-10H,2H2,(H,7,8)(H2,11,12,13)/t5-,6+/m1/s1 |
|---|
| InChI Key | HFYBTHCYPKEDQQ-RITPCOANSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Monoalkyl phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monoalkyl phosphate
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9100000000-d7621780646907150961 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-3902000000-672dc9789ba9dac4f90b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1590000000-995459a9c601e4f93bb9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01bm-7930000000-7a1fc0321a5a26cb3641 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9100000000-233300ba036335dbdb36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002s-9160000000-586419351fea64eb6eaa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-4b73b23e7fb5e36e8b12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-3234231a277bb5d275a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0076-0930000000-9d0199b4a40acd09d950 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-6900000000-61cc16ade0d5022d7ba7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-5ce5f6fd52eba0bf6485 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002s-9070000000-6f235e806e9d50bbef56 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-773be54506d41c124bd4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-7800232852d6f8ad7663 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|