| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:33:49 UTC |
|---|
| Update Date | 2020-04-22 15:48:56 UTC |
|---|
| BMDB ID | BMDB0012184 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Adenosyl cobinamide phosphate |
|---|
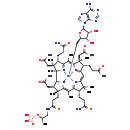
| Description | Adenosyl cobinamide phosphate belongs to the class of organic compounds known as metallotetrapyrroles. These are polycyclic compounds containing a tetrapyrrole skeleton combined with a metal atom. Adenosyl cobinamide phosphate exists in all living organisms, ranging from bacteria to humans. Based on a literature review very few articles have been published on Adenosyl cobinamide phosphate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Adenosyl cobinamide phosphoric acid | Generator | | Adenosylcobinamide phosphoric acid | HMDB | | Adenosyl cobinamide phosphate | ChEBI |
|
|---|
| Chemical Formula | C58H85CoN16O14P |
|---|
| Average Molecular Weight | 1320.3013 |
|---|
| Monoisotopic Molecular Weight | 1319.550078214 |
|---|
| IUPAC Name | (3R,4S,5S,9S,10S,15S,19R,20R,21R)-1-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}-5,10,15-tris(2-carbamoylethyl)-4,9,20-tris(carbamoylmethyl)-3,4,7,9,14,14,17,19-octamethyl-19-(2-{[(2R)-2-(phosphonooxy)propyl]carbamoyl}ethyl)-2lambda5,22,23lambda5,24lambda5-tetraaza-1-cobaltaoctacyclo[11.9.1.1^{1,8}.0^{2,6}.0^{3,21}.0^{16,23}.0^{18,22}.0^{11,24}]tetracosa-2(6),7,11(24),12,16(23),17-hexaene-2,23,24-tris(ylium)-1,1-diuide |
|---|
| Traditional Name | (3R,4S,5S,9S,10S,15S,19R,20R,21R)-1-{[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}-5,10,15-tris(2-carbamoylethyl)-4,9,20-tris(carbamoylmethyl)-3,4,7,9,14,14,17,19-octamethyl-19-(2-{[(2R)-2-(phosphonooxy)propyl]carbamoyl}ethyl)-2lambda5,22,23lambda5,24lambda5-tetraaza-1-cobaltaoctacyclo[11.9.1.1^{1,8}.0^{2,6}.0^{3,21}.0^{16,23}.0^{18,22}.0^{11,24}]tetracosa-2(6),7,11(24),12,16(23),17-hexaene-2,23,24-tris(ylium)-1,1-diuide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@H](CNC(=O)CC[C@]1(C)[C@@H](CC(N)=O)[C@H]2N3C1=C(C)C1=[N+]4C(=CC5=[N+]6C(=C(C)C7=[N+]([C@]2(C)[C@@](C)(CC(N)=O)[C@@H]7CCC(N)=O)[Co--]346C[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@](C)(CC(N)=O)[C@@H]5CCC(N)=O)C(C)(C)[C@@H]1CCC(N)=O)OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C48H74N11O11P.C10H12N5O3.Co/c1-23(70-71(67,68)69)22-55-38(66)16-17-45(6)29(18-35(52)63)43-48(9)47(8,21-37(54)65)28(12-15-34(51)62)40(59-48)25(3)42-46(7,20-36(53)64)26(10-13-32(49)60)30(56-42)19-31-44(4,5)27(11-14-33(50)61)39(57-31)24(2)41(45)58-43;1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15;/h19,23,26-29,43H,10-18,20-22H2,1-9H3,(H16,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69);2-4,6-7,10,16-17H,1H2,(H2,11,12,13);/q;;+2/p-1/t23-,26-,27-,28-,29+,43-,45-,46+,47+,48+;4-,6-,7-,10-;/m11./s1 |
|---|
| InChI Key | MQCMBMUJJHSGIF-QMUWONGRSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as metallotetrapyrroles. These are polycyclic compounds containing a tetrapyrrole skeleton combined with a metal atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Metallotetrapyrroles |
|---|
| Direct Parent | Metallotetrapyrroles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Metallotetrapyrrole skeleton
- 5'-deoxyribonucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Phosphoethanolamine
- Aminopyrimidine
- Monoalkyl phosphate
- Phosphoric acid ester
- Pyrimidine
- Organic phosphoric acid derivative
- N-substituted imidazole
- Alkyl phosphate
- Monosaccharide
- Fatty amide
- Fatty acyl
- Imidolactam
- Azole
- Heteroaromatic compound
- Imidazole
- Pyrrolidine
- Tetrahydrofuran
- Pyrroline
- Amino acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Primary carboxylic acid amide
- 1,2-diol
- Azacycle
- Oxacycle
- Organic metal salt
- Carboxylic acid derivative
- Organic transition metal salt
- Metalloheterocycle
- Organometallic compound
- Organic transition metal moeity
- Organonitrogen compound
- Transition metal alkyl
- Organic salt
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Carbonyl group
- Primary amine
- Organic oxide
- Alcohol
- Amine
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-1492000000-cd3da96c47e731cd382e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1951000000-6afc304e19ecce421bfe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3930000000-a2544aa4be58bc1ea4f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0089000000-e18b5ef096db2972b93e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0h99-0981000000-a66bdec455b06e994738 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-1910000000-2c6dbf19ff223f6e331a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|