| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:22:39 UTC |
|---|
| Update Date | 2020-04-22 15:45:53 UTC |
|---|
| BMDB ID | BMDB0011672 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Aflatoxin B1 dialcohol |
|---|
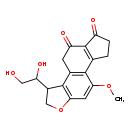
| Description | Aflatoxin B1 dialcohol belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. Based on a literature review a small amount of articles have been published on Aflatoxin B1 dialcohol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| AFB1 dialcohol | HMDB |
|
|---|
| Chemical Formula | C18H18O6 |
|---|
| Average Molecular Weight | 330.3319 |
|---|
| Monoisotopic Molecular Weight | 330.110338308 |
|---|
| IUPAC Name | 3-(1,2-dihydroxyethyl)-8-methoxy-5-oxatetracyclo[7.7.0.0²,⁶.0¹⁰,¹⁴]hexadeca-1(9),2(6),7,10(14)-tetraene-13,15-dione |
|---|
| Traditional Name | 3-(1,2-dihydroxyethyl)-8-methoxy-5-oxatetracyclo[7.7.0.0²,⁶.0¹⁰,¹⁴]hexadeca-1(9),2(6),7,10(14)-tetraene-13,15-dione |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=CC2=C(C(CO2)C(O)CO)C2=C1C1=C(C(=O)CC1)C(=O)C2 |
|---|
| InChI Identifier | InChI=1S/C18H18O6/c1-23-14-5-15-17(10(7-24-15)13(22)6-19)9-4-12(21)18-8(16(9)14)2-3-11(18)20/h5,10,13,19,22H,2-4,6-7H2,1H3 |
|---|
| InChI Key | QEIDPNWKOZPLQZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthofuran
- Naphthalene
- Coumaran
- Anisole
- Alkyl aryl ether
- Benzenoid
- Secondary alcohol
- Ketone
- 1,2-diol
- Oxacycle
- Ether
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0il1-3095000000-16ef5315b7ab6df146bc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05di-3101900000-c0f3af13cd96b13d1015 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0039000000-d8a6984403c7ad8b5c01 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ed-4094000000-5948fc0ff620c7856cc4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o1-5190000000-a1f4683c20837362b917 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0029000000-77d4b6f4f9f8a6b2e52f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02vj-1095000000-6762670b1cd34962f95a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-2090000000-194a078730ef606ab2dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0059000000-c32795aa45a9bcef9681 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-0059000000-5f5d5c2f0688199f5ad9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-0095000000-087e1cea9acd8977b36a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0049000000-9a86ca58dcf438f3b2cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-0090000000-6f189f4bbe7fc2b09e64 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fur-0091000000-9a41cedfbfb6cfb0672b | View in MoNA |
|---|
|
|---|