| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:29 UTC |
|---|
| Update Date | 2020-05-11 20:25:03 UTC |
|---|
| BMDB ID | BMDB0010330 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Cholesterol glucuronide |
|---|
| Description | Cholesterol glucuronide belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. Based on a literature review a significant number of articles have been published on Cholesterol glucuronide. |
|---|
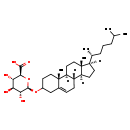
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cholest-5-en-3beta-yl beta-D-glucopyranosiduronic acid | HMDB | | Cholest-5-en-3beta-yl beta-delta-glucopyranosiduronic acid | HMDB | | Cholesterol glucopyranosiduronate | HMDB | | Cholesterol glucosiduronate | HMDB | | Cholesteryl beta-D-glucosiduronic acid | HMDB | | Cholesteryl beta-D-glucuronide | HMDB | | Cholesteryl beta-delta-glucosiduronic acid | HMDB | | Cholesteryl-glucopyranosiduronic acid | HMDB | | Cholesterylglucopyranosiduronic acid | HMDB | | 3-O-beta-D-Glucopyranuronosyl cholesterol | MeSH, HMDB | | (2S,3S,4S,5R,6R)-6-{[(1S,2R,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylate | Generator | | Cholesterol glucuronide | MeSH |
|
|---|
| Chemical Formula | C33H54O7 |
|---|
| Average Molecular Weight | 562.7777 |
|---|
| Monoisotopic Molecular Weight | 562.386954082 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(1S,2R,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-6-{[(1S,2R,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | 17435-78-8 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4CC(CC[C@]4(C)[C@@]3([H])CC[C@]12C)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O)[C@H](C)CCCC(C)C |
|---|
| InChI Identifier | InChI=1S/C33H54O7/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(13-15-32(20,4)25(22)14-16-33(23,24)5)39-31-28(36)26(34)27(35)29(40-31)30(37)38/h9,18-19,21-29,31,34-36H,6-8,10-17H2,1-5H3,(H,37,38)/t19-,21?,22+,23-,24+,25+,26+,27+,28-,29+,31-,32+,33-/m1/s1 |
|---|
| InChI Key | IJLBJBCDNYOWPJ-BBPBCBHPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpene glycoside
- Steroid-glucuronide-skeleton
- Cholesterol
- Cholestane-skeleton
- Diterpenoid
- Delta-5-steroid
- Terpene glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Pyran
- Hydroxy acid
- Monosaccharide
- Oxane
- Secondary alcohol
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0035-7307490000-bf58af3b5090641f2b4a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-06di-8416119000-64a938d78592fb23c354 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Cholesterol glucuronide,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-029j-0009060000-f163d11ac52f85bebc1b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-1109000000-9b6793f4abea11fa40b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2309000000-f616dc38fcc5c5d34e0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03y0-1207190000-d749b17183b79d147d5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1209020000-446843a5efb09fef2b03 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-4109000000-0782320c2feb2515746e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000090000-c381135fb7279c597e43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aos-5297020000-157fc21c3b4fd59bd4be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9721200000-08dbeda3826e05d92e8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-c1f737b4e91df5056283 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-2202190000-d476e69fac751e5e871c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9204630000-fb068e954db2e5a173b8 | View in MoNA |
|---|
|
|---|