| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:04:12 UTC |
|---|
| Update Date | 2020-05-11 20:42:04 UTC |
|---|
| BMDB ID | BMDB0010316 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Acetaminophen glucuronide |
|---|
| Description | Acetaminophen glucuronide, also known as 4-glucuronosidoacetanilide or deethylphenacetin glucuronide, belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. Acetaminophen glucuronide is an extremely weak basic (essentially neutral) compound (based on its pKa). Acetaminophen glucuronide, with regard to humans, has been linked to the inborn metabolic disorder beta-thalassemia. |
|---|
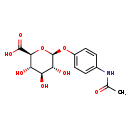
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Paracetamol glucuronide | ChEBI | | 4-(Acetylamino)phenyl beta-D-glucopyranosiduronic acid | HMDB | | 4-(Acetylamino)phenyl beta-delta-glucopyranosiduronic acid | HMDB | | 4-Acetamidophenol glucuronide | HMDB | | 4-Acetamidophenyl b-D-glucopyranosiduronic acid | HMDB | | 4-Acetamidophenyl b-delta-glucopyranosiduronic acid | HMDB | | 4-Acetamidophenyl beta-D-glucopyranosiduronic acid | HMDB | | 4-Acetamidophenyl beta-delta-glucopyranosiduronic acid | HMDB | | p-Acetamidophenyl β-D-glucuronide | HMDB | | 4-(Acetylamino)phenyl β-D-glucopyranosiduronic acid | HMDB | | 4-Glucuronosidoacetanilide | HMDB | | 4-Hydroxyacetanilide glucuronide | HMDB | | Acetanilide glucuronide | HMDB | | 4'-(Glucuronosyloxy)-acetanilide | HMDB | | Deethylphenacetin glucuronide | HMDB | | N-Acetyl-4-aminophenol glucuronide | HMDB | | N-Acetyl-4-glucuronosidoaniline | HMDB | | N-Acetyl-p-aminophenyl glucuronide | HMDB | | Paracetamol O-glucuronide | HMDB | | Paracetamol β-glucuronide | HMDB | | Paracetamol beta-glucuronide | HMDB | | p-Acetamidophenyl D-glucosiduronic acid | HMDB | | p-Acetamidophenyl glucosiduronic acid | HMDB | | p-Acetamidophenyl glucuronide | HMDB | | PCM-g | HMDB | | p-Acetamidophenylglucuronide | HMDB | | Acetaminophen glucuronide | HMDB |
|
|---|
| Chemical Formula | C14H17NO8 |
|---|
| Average Molecular Weight | 327.2867 |
|---|
| Monoisotopic Molecular Weight | 327.095416525 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-6-(4-acetamidophenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | acetaminophen glucuronide |
|---|
| CAS Registry Number | 16110-10-4 |
|---|
| SMILES | CC(=O)NC1=CC=C(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C14H17NO8/c1-6(16)15-7-2-4-8(5-3-7)22-14-11(19)9(17)10(18)12(23-14)13(20)21/h2-5,9-12,14,17-19H,1H3,(H,15,16)(H,20,21)/t9-,10-,11+,12-,14+/m0/s1 |
|---|
| InChI Key | IPROLSVTVHAQLE-BYNIDDHOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- O-glycosyl compound
- Phenoxy compound
- Phenol ether
- Beta-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Monosaccharide
- Benzenoid
- Pyran
- Oxane
- Secondary alcohol
- Acetal
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0961000000-dcaaa03ec12ac3e8bc7b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0961000000-dcaaa03ec12ac3e8bc7b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-9352000000-77fed48f2ac24b73fab8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0udi-4100349000-5176b6b37f6857b7f999 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-03k9-0900000000-5e02cd6d491f4b1cf8f5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-00di-0900000000-acce7f7634727584b435 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-000i-0922000000-d3ea032d2b2a786b49a4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-7bb565a0815d9e8681c4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0w29-2900000000-8029b9ba6317a5c7bacb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1902000000-63bf36b7f8481253b041 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-03di-3901000000-ac725135cde5a222d64a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-9800000000-d0386babb34ac3708373 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-014l-9200000000-dc0cedc02a918704f9c2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-0udi-0907000000-cc6b4eeb1854a02b091e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0ik9-2900000000-f45589acb1254525eedc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-03di-4900000000-8f1b83b451250f90fa5f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0xxr-3900000000-560c53f10968963e3a63 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-0udi-0907000000-ab44d212f9ebe70f73e6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0zfr-9800000000-16b1e116a6a82ae143a1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-7900000000-0106d7d985bdfc7ce4ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0902000000-232646fdc95c354d8ef2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0901000000-24d1372940103501af9d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0udi-6900000000-65f843147decea0cfc44 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0w4i-0946000000-4049f1415ff9576f5924 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-0910000000-a1b732d82b46888499a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc0-2900000000-7fe5c0cbd17367744596 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fc0-2968000000-a253cd8c945d814768e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2931000000-8678b1c192068d44639b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pc0-3900000000-6e974e4924ffcc0ae778 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|