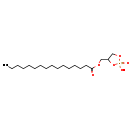| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:28:33 UTC |
|---|
| Update Date | 2020-05-11 18:26:04 UTC |
|---|
| BMDB ID | BMDB0007003 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | CPA(16:0/0:0) |
|---|
| Description | CPA(16:0/0:0) belongs to the class of organic compounds known as 1-acyl-sn-glycerol-2,3-cyclic-phosphates. These are monoacylglycerophosphates consisting of a sn-glycerol 2,3-cyclic phosphate that carries an acyl group at the 1-position. Based on a literature review very few articles have been published on CPA(16:0/0:0). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Hexadecanoyl-cyclophosphatidic acid | HMDB | | 1-Palmitoyl-glycero-3-cyclophosphate | HMDB | | CPA(16:0) | HMDB | | Cyclic phosphatidic acid(16:0) | HMDB | | Cyclic phosphatidic acid(16:0/0:0) | HMDB | | (2-Hydroxy-2-oxo-1,3,2λ⁵-dioxaphospholan-4-yl)methyl hexadecanoic acid | Generator |
|
|---|
| Chemical Formula | C19H37O6P |
|---|
| Average Molecular Weight | 392.4672 |
|---|
| Monoisotopic Molecular Weight | 392.232775428 |
|---|
| IUPAC Name | (2-hydroxy-2-oxo-1,3,2λ⁵-dioxaphospholan-4-yl)methyl hexadecanoate |
|---|
| Traditional Name | (2-hydroxy-2-oxo-1,3,2λ⁵-dioxaphospholan-4-yl)methyl hexadecanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)OCC1COP(O)(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C19H37O6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19(20)23-16-18-17-24-26(21,22)25-18/h18H,2-17H2,1H3,(H,21,22) |
|---|
| InChI Key | WLYRJURLXSFXRK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-acyl-sn-glycerol-2,3-cyclic-phosphates. These are monoacylglycerophosphates consisting of a sn-glycerol 2,3-cyclic phosphate that carries an acyl group at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphates |
|---|
| Direct Parent | 1-acyl-sn-glycerol-2,3-cyclic-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-acyl-sn-glycerol-2,3-cyclic-phosphate
- Fatty acid ester
- Organic phosphoric acid derivative
- Fatty acyl
- 1,3_dioxaphospholane
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-2941000000-a7754b3d4d0b019ecd15 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-5859000000-3e458c8ea3e85d9bbe45 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-7961000000-376022ff01155f430544 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000m-6920000000-77c63fe8998476b2c35b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4u-0195000000-72b269827d76881aa8f8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-2391000000-870f830b62d92cff6547 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9020000000-63a5c62523e8cc061b89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000x-0619000000-1210e56060ec93566a5d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4590000000-d96abd5e1fa5ad56efb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9520000000-d31380c77196dfd340b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-3709000000-85e4fffd82d840924cb5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-5901000000-3ff44f491b8a9d6e8a74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9300000000-00f6f81aa8aba1e3fef5 | View in MoNA |
|---|
|
|---|