| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:26:03 UTC |
|---|
| Update Date | 2020-04-22 15:18:36 UTC |
|---|
| BMDB ID | BMDB0006794 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
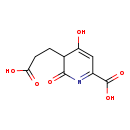
| Common Name | 5-(2'-Carboxyethyl)-4,6-Dihydroxypicolinate |
|---|
| Description | 5-(2'-Carboxyethyl)-4,6-Dihydroxypicolinate belongs to the class of organic compounds known as dihydropyridinecarboxylic acids and derivatives. Dihydropyridinecarboxylic acids and derivatives are compounds containing a dihydropyridine moiety bearing a carboxylic acid group. Based on a literature review very few articles have been published on 5-(2'-Carboxyethyl)-4,6-Dihydroxypicolinate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-(2'-Carboxyethyl)-4,6-dihydroxypicolinic acid | Generator | | 5-(beta-Carboxyethyl)-4,6-dihydroxypicolinate | HMDB | | 5-(2-Carboxyethyl)-4-hydroxy-6-oxo-5,6-dihydropyridine-2-carboxylate | Generator, HMDB |
|
|---|
| Chemical Formula | C9H9NO6 |
|---|
| Average Molecular Weight | 227.1709 |
|---|
| Monoisotopic Molecular Weight | 227.042987025 |
|---|
| IUPAC Name | 5-(2-carboxyethyl)-4-hydroxy-6-oxo-5,6-dihydropyridine-2-carboxylic acid |
|---|
| Traditional Name | 5-(2-carboxyethyl)-4-hydroxy-6-oxo-5H-pyridine-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)CCC1C(O)=CC(=NC1=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H9NO6/c11-6-3-5(9(15)16)10-8(14)4(6)1-2-7(12)13/h3-4,11H,1-2H2,(H,12,13)(H,15,16) |
|---|
| InChI Key | PAKGMMKOUIXEKV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydropyridinecarboxylic acids and derivatives. Dihydropyridinecarboxylic acids and derivatives are compounds containing a dihydropyridine moiety bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Hydropyridines |
|---|
| Direct Parent | Dihydropyridinecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyridinecarboxylic acid derivative
- Dicarboxylic acid or derivatives
- N-acylimine
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Enol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lu-5920000000-34db0eebe1998991028c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00b9-6139200000-372ff3e14b1d8b8604e0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0490000000-b25973ab1bfbe54d9520 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-0920000000-95ceef583dd5d9905638 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fki-8900000000-ea0ce6faafbf07ed0e5f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-056r-0390000000-a8eaed9d1539e12b41ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bw9-1940000000-40880fc7ca5263df22be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9500000000-b1845995bab276ba2f4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03gi-0950000000-4de548e96ed211ba61e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q0-0900000000-aa196a883e346a1513bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p6-4900000000-6994f2501e6d5238a198 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0690000000-168c424843335c4e4891 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-0910000000-7595296cb7f7b64e62be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lb-6900000000-f6f1a3bbadbb719fbe2b | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|