| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:23:15 UTC |
|---|
| Update Date | 2020-04-22 15:17:49 UTC |
|---|
| BMDB ID | BMDB0006552 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Aflatoxin B1 |
|---|
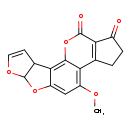
| Description | Aflatoxin B1 belongs to the class of organic compounds known as difurocoumarocyclopentenones. These are polycyclic aromatic compounds containing a cyclopenten-2-one ring fused to the coumarin moiety of the difurocoumarin skeleton. Thus, aflatoxin B1 is considered to be an aflatoxin lipid molecule. Aflatoxin B1 is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Aflatoxin B1 is formally rated as a carcinogen (by IARC 1) and is also a potentially toxic compound. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| AFB1 | HMDB | | AFBI | HMDB | | HSDB-3453 | MeSH, HMDB | | Aflatoxin b | MeSH, HMDB | | Aflatoxin b1, (6ar-cis)-isomer, 14C-labeled | MeSH, HMDB | | Aflatoxin b1, (6ar-cis)-isomer, 2H-labeled | MeSH, HMDB | | HSDB 3453 | MeSH, HMDB | | Aflatoxin b1 dihydrochloride, (6ar-cis)-isomer | MeSH, HMDB | | Aflatoxin b1, cis(+,-)-isomer | MeSH, HMDB | | Aflatoxin b1, (6ar-cis)-isomer, 3H-labeled | MeSH, HMDB |
|
|---|
| Chemical Formula | C17H12O6 |
|---|
| Average Molecular Weight | 312.2736 |
|---|
| Monoisotopic Molecular Weight | 312.063388116 |
|---|
| IUPAC Name | 11-methoxy-6,8,19-trioxapentacyclo[10.7.0.0²,⁹.0³,⁷.0¹³,¹⁷]nonadeca-1,4,9,11,13(17)-pentaene-16,18-dione |
|---|
| Traditional Name | aflatoxin |
|---|
| CAS Registry Number | 1162-65-8 |
|---|
| SMILES | COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C2C3C=COC3OC2=C1 |
|---|
| InChI Identifier | InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3 |
|---|
| InChI Key | OQIQSTLJSLGHID-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as difurocoumarocyclopentenones. These are polycyclic aromatic compounds containing a cyclopenten-2-one ring fused to the coumarin moiety of the difurocoumarin skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Difurocoumarocyclopentenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Difurocoumarocyclopentenone
- Benzopyran
- 1-benzopyran
- Coumaran
- Anisole
- Aryl alkyl ketone
- Aryl ketone
- Alkyl aryl ether
- Pyranone
- Pyran
- Benzenoid
- Heteroaromatic compound
- Dihydrofuran
- Lactone
- Ketone
- Ether
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|