| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:22:46 UTC |
|---|
| Update Date | 2020-04-22 15:17:40 UTC |
|---|
| BMDB ID | BMDB0006512 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Mercaptolactate-cysteine disulfide |
|---|
| Description | 3-Mercaptolactate-cysteine disulfide, also known as S-(2-hydroxy-2-carboxyethylthio)cysteine, belongs to the class of organic compounds known as l-cysteine-s-conjugates. L-cysteine-S-conjugates are compounds containing L-cysteine where the thio-group is conjugated. Based on a literature review a small amount of articles have been published on 3-Mercaptolactate-cysteine disulfide. |
|---|
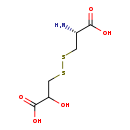
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Mercaptolactate-cysteine disulphide | Generator | | 3-Mercaptolactic acid-cysteine disulfide | Generator | | 3-Mercaptolactic acid-cysteine disulphide | Generator | | S-(2-Hydroxy-2-carboxyethylthio)cysteine | MeSH | | beta-Mercaptolactate cysteine disulfide | MeSH | | 3-((2-Carboxy-2-hydroxyethyl)dithio)-L-alanine | HMDB | | Hcetc | HMDB | | 3-{[(2R)-2-amino-2-carboxyethyl]disulfanyl}-2-hydroxypropanoate | Generator, HMDB | | 3-{[(2R)-2-amino-2-carboxyethyl]disulphanyl}-2-hydroxypropanoate | Generator, HMDB | | 3-{[(2R)-2-amino-2-carboxyethyl]disulphanyl}-2-hydroxypropanoic acid | Generator, HMDB | | 3-Mercaptolactate-cysteine disulfide | MeSH |
|
|---|
| Chemical Formula | C6H11NO5S2 |
|---|
| Average Molecular Weight | 241.285 |
|---|
| Monoisotopic Molecular Weight | 241.007863847 |
|---|
| IUPAC Name | 3-{[(2R)-2-amino-2-carboxyethyl]disulfanyl}-2-hydroxypropanoic acid |
|---|
| Traditional Name | hcetc |
|---|
| CAS Registry Number | 18841-42-4 |
|---|
| SMILES | N[C@@H](CSSCC(O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H11NO5S2/c7-3(5(9)10)1-13-14-2-4(8)6(11)12/h3-4,8H,1-2,7H2,(H,9,10)(H,11,12)/t3-,4?/m0/s1 |
|---|
| InChI Key | MAFDYIDMXCXBRB-WUCPZUCCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-cysteine-s-conjugates. L-cysteine-S-conjugates are compounds containing L-cysteine where the thio-group is conjugated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-cysteine-S-conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-cysteine-s-conjugate
- Alpha-amino acid
- L-alpha-amino acid
- Alpha-hydroxy acid
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Monosaccharide
- Amino acid
- Dialkyldisulfide
- Organic disulfide
- Secondary alcohol
- Carboxylic acid
- Sulfenyl compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Alcohol
- Carbonyl group
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Organosulfur compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0096-9300000000-68e01abf228e831fed45 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-9533100000-d4f0ecb6cd097f65b9dd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006w-1960000000-f56bf92526e8f726becf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ds-3910000000-c2b2312a5af041580ab5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-7900000000-a49dadf9d375535e0dce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udl-4950000000-6b12c4c4f730b800e73b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-6900000000-ec3b44873b06061157f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-9200000000-c80fb1c7de58ac714124 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0g4i-2900000000-368301025d75cd74dd85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0230-9500000000-6b2727915ff92bbf8cf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-047m-9100000000-3c559c5d78dfba6464a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0890000000-8babd75cca365d589ef6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-6900000000-6b7f3b667ac4477205d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-e35fd1cdef28827b87aa | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|