| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:21:37 UTC |
|---|
| Update Date | 2020-05-11 20:41:28 UTC |
|---|
| BMDB ID | BMDB0006335 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Beta-tocopherol |
|---|
| Description | beta-Tocopherol, also known as β-tocopherol or 5,8-dimethyltocol, belongs to the class of organic compounds known as tocopherols. These are vitamin E derivatives containing a saturated trimethyltridecyl chain attached to the carbon C6 atom of a benzopyran ring system. The differ from tocotrienols that contain an unsaturated trimethyltrideca-3,7,11-trien-1-yl chain. Thus, beta-tocopherol is considered to be a quinone. Based on a literature review a significant number of articles have been published on beta-Tocopherol. |
|---|
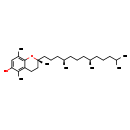
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2R)-2,5,8-Trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol | ChEBI | | (2R)-3,4-Dihydro-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol | ChEBI | | (2R,4'r,8'r)-beta-Tocopherol | ChEBI | | (R,R,R)-beta-Tocopherol | ChEBI | | 5,8-Dimethyltocol | ChEBI | | RRR-beta-Tocopherol | ChEBI | | (2R,4'r,8'r)-b-Tocopherol | Generator | | (2R,4'r,8'r)-Β-tocopherol | Generator | | (R,R,R)-b-Tocopherol | Generator | | (R,R,R)-Β-tocopherol | Generator | | RRR-b-Tocopherol | Generator | | RRR-Β-tocopherol | Generator | | b-Tocopherol | Generator | | Β-tocopherol | Generator | | beta Tocopherol | HMDB | | 3,4-Dihydro-2,5,8-trimethyl-2-(4,8,12- trimethyltridecyl)-2H-1-benzopyran-6-ol | HMDB | | b-Tocopherol skeleton | HMDB | | Β-tocopherol skeleton | HMDB | | Cumotocopherol | HMDB | | DL-beta-Tocopherol | HMDB | | DL-Β-tocopherol | HMDB | | Neotocopherol | HMDB | | p-Xylotocopherol | HMDB | | beta-Tocopherol | MeSH | | 2,5,8-Trimethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol | HMDB |
|
|---|
| Chemical Formula | C28H48O2 |
|---|
| Average Molecular Weight | 416.6795 |
|---|
| Monoisotopic Molecular Weight | 416.36543078 |
|---|
| IUPAC Name | (2R)-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol |
|---|
| Traditional Name | β-tocopherol |
|---|
| CAS Registry Number | 148-03-8 |
|---|
| SMILES | CC(C)CCC[C@@H](C)CCC[C@@H](C)CCC[C@]1(C)CCC2=C(C)C(O)=CC(C)=C2O1 |
|---|
| InChI Identifier | InChI=1S/C28H48O2/c1-20(2)11-8-12-21(3)13-9-14-22(4)15-10-17-28(7)18-16-25-24(6)26(29)19-23(5)27(25)30-28/h19-22,29H,8-18H2,1-7H3/t21-,22-,28-/m1/s1 |
|---|
| InChI Key | WGVKWNUPNGFDFJ-DQCZWYHMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tocopherols. These are vitamin E derivatives containing a saturated trimethyltridecyl chain attached to the carbon C6 atom of a benzopyran ring system. The differ from tocotrienols that contain an unsaturated trimethyltrideca-3,7,11-trien-1-yl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Tocopherols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tocopherol
- Diterpenoid
- 1-benzopyran
- Benzopyran
- Chromane
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | < 25 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-00di-1490200000-f75de727e16d87c65fba | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00di-1490200000-f75de727e16d87c65fba | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0lyo-6954200000-ffcf92e6a6e2f34dd922 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-5644900000-b5341f1bdc151c1a796b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-EBEB (JMS-HX/HX 110A, JEOL) , Positive | splash10-0gb9-3900600000-10f458ddcb7e7aa4df84 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 29V, negative | splash10-0f6t-0900600000-9a11a437c645252a72a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0842900000-82c505b0548997aace83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1910000000-1b1a0fe2f352daa49e2c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-4920000000-b63881a7743c06bdea15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0100900000-2345f13b44d6c70d81e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0912800000-4e68210f69fcd3f6c374 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kk-0914000000-f6f5255b874c4fc7f4f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-a74b65a822c3cc7625b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0201900000-2dcdf4f502cc6e57342d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-024m-2913100000-fb9e3e53715df460db95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-3113900000-16d3b372ee248c26f313 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-9213100000-65be5213a11752835e85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052g-9400000000-947da441c6df0de93bed | View in MoNA |
|---|
|
|---|