| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:15 UTC |
|---|
| Update Date | 2020-04-22 15:16:52 UTC |
|---|
| BMDB ID | BMDB0006117 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | APGPR Enterostatin |
|---|
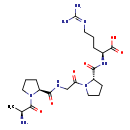
| Description | APGPR Enterostatin, also known as ala-pro-gly-pro-arg or APGPR, belongs to the class of organic compounds known as benzoyl derivatives. These are organic compounds containing an acyl moiety of benzoic acid with the formula (C6H5CO-). Based on a literature review a small amount of articles have been published on APGPR Enterostatin. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ala-pro-gly-pro-arg | MeSH | | APGPR | MeSH | | Procolipase activation peptide | MeSH | | Alanyl-prolyl-glycyl-prolyl-arginine | MeSH | | H-Ala-pro-gly-pro-arg-OH | HMDB | | L-Alanyl-L-prolylglycyl-L-prolyl-L-arginine | HMDB | | N2-[1-[N-(1-L-Alanyl-L-prolyl)glycyl]-L-prolyl] L-arginine | HMDB | | (2S)-2-({[(2S)-1-[2-({[(2S)-1-[(2S)-2-aminopropanoyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)acetyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-5-carbamimidamidopentanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C21H36N8O6 |
|---|
| Average Molecular Weight | 496.5605 |
|---|
| Monoisotopic Molecular Weight | 496.275780924 |
|---|
| IUPAC Name | (2S)-2-{[(2S)-1-(2-{[(2S)-1-[(2S)-2-aminopropanoyl]pyrrolidin-2-yl]formamido}acetyl)pyrrolidin-2-yl]formamido}-5-[(diaminomethylidene)amino]pentanoic acid |
|---|
| Traditional Name | enterostatin |
|---|
| CAS Registry Number | 117830-79-2 |
|---|
| SMILES | C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C21H36N8O6/c1-12(22)19(33)29-10-4-6-14(29)17(31)26-11-16(30)28-9-3-7-15(28)18(32)27-13(20(34)35)5-2-8-25-21(23)24/h12-15H,2-11,22H2,1H3,(H,26,31)(H,27,32)(H,34,35)(H4,23,24,25)/t12-,13-,14-,15-/m0/s1 |
|---|
| InChI Key | ITZMJCSORYKOSI-AJNGGQMLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoyl derivatives. These are organic compounds containing an acyl moiety of benzoic acid with the formula (C6H5CO-). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoyl derivatives |
|---|
| Direct Parent | Benzoyl derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoyl
- Benzaldehyde
- Aryl-aldehyde
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aldehyde
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9331200000-e17547b3084d5e946ba7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-9321020000-dc00034c773e0eacec8a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-3822900000-bf575ffecf20de104707 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-7931000000-04d78f53a54fd2c77bcc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00fr-9620000000-76f0df5682dda6f687b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1000900000-3a5434deb4d11d3ee6a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-6432900000-ee05f2b260b1e79be45b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9310100000-09ae0ee586b3d8ea2b85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-2000900000-a65ea133a6df00df3087 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-2101900000-f9459d79d1f036613677 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9320100000-b44a31e7e4fb479a6aa4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-6a40962b10f4881333f8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000t-2120900000-adc7331f3479a2c9aed6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-4390000000-98814835c5c1e558df62 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|