| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:54 UTC |
|---|
| Update Date | 2020-04-22 15:16:45 UTC |
|---|
| BMDB ID | BMDB0006040 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N2-Methylguanine |
|---|
| Description | N2-Methylguanine belongs to the class of organic compounds known as hypoxanthines. Hypoxanthines are compounds containing the purine derivative 1H-purin-6(9H)-one. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. Based on a literature review a significant number of articles have been published on N2-Methylguanine. |
|---|
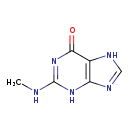
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,7-Dihydro-2-(methylamino)-6H-purin-6-one | ChEBI | | 2-Methylamino-6-oxopurine | ChEBI | | 2-Methylguanine | ChEBI | | N-Methyl-guanine | ChEBI | | 1, 7-dihydro-2-(methylamino)-6H-Purin-6-one | HMDB | | 2-(methylamino)-9H-Purin-6-ol | HMDB | | 2-methylamino-1,9-dihydro-Purin-6-one | HMDB | | 2-monomethylamino-6-Hydroxypurine | HMDB | | 6-Hydroxy-2-methylamino-purine | HMDB | | 6-Hydroxy-2-methylaminopurine | HMDB | | 7-Methylguanine | MeSH, HMDB | | N7-Me-g | MeSH, HMDB | | 7-Methylguanine, 14C-labeled | MeSH, HMDB | | N(2)-Methylguanine | MeSH, HMDB | | N7-Methylguanine | MeSH, HMDB | | N2-Methylguanine | MeSH |
|
|---|
| Chemical Formula | C6H7N5O |
|---|
| Average Molecular Weight | 165.1527 |
|---|
| Monoisotopic Molecular Weight | 165.065059871 |
|---|
| IUPAC Name | 2-(methylamino)-6,7-dihydro-3H-purin-6-one |
|---|
| Traditional Name | 2-(methylamino)-3,7-dihydropurin-6-one |
|---|
| CAS Registry Number | 10030-78-1 |
|---|
| SMILES | CNC1=NC(=O)C2=C(N1)N=CN2 |
|---|
| InChI Identifier | InChI=1S/C6H7N5O/c1-7-6-10-4-3(5(12)11-6)8-2-9-4/h2H,1H3,(H3,7,8,9,10,11,12) |
|---|
| InChI Key | SGSSKEDGVONRGC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hypoxanthines. Hypoxanthines are compounds containing the purine derivative 1H-purin-6(9H)-one. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Hypoxanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 6-oxopurine
- Hypoxanthine
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Imidazole
- Azole
- Azacycle
- Secondary amine
- Organic nitrogen compound
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00li-2900000000-539ed57cd0169be157e1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-c5b4249fffca3d4d371f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-e0e61dd036c1eeb8c7cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-9700000000-bc35e49ab2b1f81d19f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-18f6ed24aee9629d2b71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dr-1900000000-3647b625be48894ded97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9200000000-eb014bf17054735890c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-11e5406fb2e0acd600c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03el-1900000000-3dab99342f0796ffff0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-568aee5531b232c43016 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-7f20cf9333bbb1eb98e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1900000000-2641d6f2b8a568415740 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9300000000-58b99541aa08fb42a838 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|