| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:11 UTC |
|---|
| Update Date | 2020-04-22 15:16:32 UTC |
|---|
| BMDB ID | BMDB0005869 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3,3'-Diiodothyronine |
|---|
| Description | 3,3'-Diiodothyronine belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review a significant number of articles have been published on 3,3'-Diiodothyronine. |
|---|
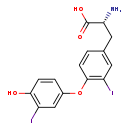
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3'-diiodo-L-Thyronine | HMDB | | 3,3'-T2 | HMDB | | 3-[4-(4-Hydroxy-3-iodophenoxy)-3-iodophenyl]-L-alanine | HMDB | | L-3-[4-(4-Hydroxy-3-iodophenoxy)-3-iodophenyl]-alanine | HMDB | | L-beta-[4-(4-Hydroxy-3-iodophenoxy)-3-iodophenyl]-alanine | HMDB | | O-(4-Hydroxy-3-iodophenyl)-3-iodo-L-tyrosine | HMDB | | O-(4-Hydroxy-3-iodophenyl)-3-iodo-tyrosine | HMDB | | O-(4-Hydroxy-3-iodophenyl)-3-iodotyrosine | HMDB | | 3,3'-Diiodothyronine, (L)-isomer | MeSH, HMDB | | 3,3'-Diiodothyronine, (L)-isomer, 125I-labeled | MeSH, HMDB | | 3,3'-Diiodothyronine, (DL)-isomer | MeSH, HMDB | | (2R)-2-Amino-3-[4-(4-hydroxy-3-iodophenoxy)-3-iodophenyl]propanoate | Generator, HMDB | | 3,3'-Diiodothyronine | MeSH |
|
|---|
| Chemical Formula | C15H13I2NO4 |
|---|
| Average Molecular Weight | 525.077 |
|---|
| Monoisotopic Molecular Weight | 524.893394749 |
|---|
| IUPAC Name | (2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3-iodophenyl]propanoic acid |
|---|
| Traditional Name | 3,3'-diiodo-L-thyronine |
|---|
| CAS Registry Number | 70-40-6 |
|---|
| SMILES | N[C@H](CC1=CC(I)=C(OC2=CC(I)=C(O)C=C2)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H13I2NO4/c16-10-7-9(2-3-13(10)19)22-14-4-1-8(5-11(14)17)6-12(18)15(20)21/h1-5,7,12,19H,6,18H2,(H,20,21)/t12-/m1/s1 |
|---|
| InChI Key | CPCJBZABTUOGNM-GFCCVEGCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Diphenylether
- Diaryl ether
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- D-alpha-amino acid
- Phenoxy compound
- 2-iodophenol
- 2-halophenol
- Phenol ether
- Iodobenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Halobenzene
- Phenol
- Aralkylamine
- Aryl iodide
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Ether
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Primary aliphatic amine
- Organohalogen compound
- Organoiodide
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-6031920000-71c3f9fd5d3525d32324 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0udr-9220062000-622a98eee44fd2a653d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000980000-858065ad3e75dc6659f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000910000-a2dcc5e08cee0881ad4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr6-0090400000-a070e8cbcc117f994f6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000090000-65d3cc7d62473f25536e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05gi-0041490000-5c480959f3bd3dc4837a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pir-8394100000-89e4e80d17cbdfd7d9e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000970000-81a694fc24040341b724 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0001900000-ad927620975a1b760382 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-0092100000-8085fd591d50843db9fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000290000-d0a979f9285e9d348d71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0229-2101950000-c313cc0aa358b2f36374 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0912000000-9f2ef8ddde02d9a68c1a | View in MoNA |
|---|
|
|---|