| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:07 UTC |
|---|
| Update Date | 2020-04-22 15:16:30 UTC |
|---|
| BMDB ID | BMDB0005844 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 8-isoprostaglandin E2 |
|---|
| Description | 8-isoprostaglandin E2, also known as 8-epi-pge2, belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. Thus, 8-isoprostaglandin E2 is considered to be an eicosanoid. Based on a literature review a significant number of articles have been published on 8-isoprostaglandin E2. |
|---|
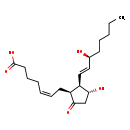
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7-[3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl]-5-heptenoic acid | ChEBI | | 8-Epi-pge2 | ChEBI | | 8-Iso-pge2 | ChEBI | | 7-[3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl]-5-heptenoate | Generator | | (5Z,8b,11a,13E,15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-Oate | HMDB | | (5Z,8b,11a,13E,15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-Oic acid | HMDB | | 8-Isoprostaglandin e2 | ChEBI |
|
|---|
| Chemical Formula | C20H32O5 |
|---|
| Average Molecular Weight | 352.4651 |
|---|
| Monoisotopic Molecular Weight | 352.224974134 |
|---|
| IUPAC Name | (5Z)-7-[(1S,2R,3R)-3-hydroxy-2-[(1E,3S)-3-hydroxyoct-1-en-1-yl]-5-oxocyclopentyl]hept-5-enoic acid |
|---|
| Traditional Name | 8-Iso-PGE2 |
|---|
| CAS Registry Number | 27415-25-4 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@H]1C\C=C/CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16-,17+,19+/m0/s1 |
|---|
| InChI Key | XEYBRNLFEZDVAW-CLQOMRTCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Prostaglandins and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Prostaglandin skeleton
- Long-chain fatty acid
- Hydroxy fatty acid
- Cyclopentanol
- Fatty acid
- Unsaturated fatty acid
- Cyclic alcohol
- Ketone
- Cyclic ketone
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a73-5395000000-02aad995307bb6a67f94 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0ufu-9302550000-70dfa502c5c46d488f7a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0019000000-f68c6b496bc66132ac05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-3197000000-5c1986771d855fb6ed90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9210000000-12fff721757996d80d14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ue9-0009000000-8f0c944be1857e336476 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-2059000000-3515e7355791e5a9219f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9430000000-8cbabd2bf835ca82e917 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-0049000000-1d1e45c7e0b65ddd683e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0097000000-dcf0f9c6213818037863 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-8590000000-6ae1006cdd7f50388698 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-3d13808f266c10b60336 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-4289000000-1d06c57536d9cf1761f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014u-9800000000-a2144167462af022d6f9 | View in MoNA |
|---|
|
|---|