| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:18:54 UTC |
|---|
| Update Date | 2020-04-22 15:16:26 UTC |
|---|
| BMDB ID | BMDB0005814 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Testosterone enanthate |
|---|
| Description | Testosterone enanthate, also known as delatestryl, belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. Based on a literature review a significant number of articles have been published on Testosterone enanthate. |
|---|
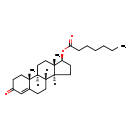
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17-((1-Oxoheptyl)oxy)androst-4-en-3-one | ChEBI | | 17-Hydroxyandrost-4-en-3-one, 17-heptanoate | ChEBI | | 17beta-Hydroxyandrost-4-en-3-one heptanoate | ChEBI | | Testosterone 17-enanthate | ChEBI | | Testosterone heptanoate | ChEBI | | Delatestryl | Kegg | | 17-Hydroxyandrost-4-en-3-one, 17-heptanoic acid | Generator | | 17b-Hydroxyandrost-4-en-3-one heptanoate | Generator | | 17b-Hydroxyandrost-4-en-3-one heptanoic acid | Generator | | 17beta-Hydroxyandrost-4-en-3-one heptanoic acid | Generator | | 17Β-hydroxyandrost-4-en-3-one heptanoate | Generator | | 17Β-hydroxyandrost-4-en-3-one heptanoic acid | Generator | | Testosterone 17-enanthic acid | Generator | | Testosterone heptanoic acid | Generator | | Testosterone enanthic acid | Generator | | 17beta-Enanthoxyandrost-4-en-3-one | HMDB | | 17beta-Hydroxyandrost-4-en-3-one enanthate | HMDB | | 4-Androsten-17beta-ol-3-one 17-enanthate | HMDB | | 4-Androsten-3-one 17beta-enanthate | HMDB | | Androgyn l.a. | HMDB | | Andropository | HMDB | | Androtardyl | HMDB | | Atlatest | HMDB | | DEA no. 4000 | HMDB | | Delatest | HMDB | | DePatestrye | HMDB | | depo-testro Med | HMDB | | Ditate | HMDB | | Durathate | HMDB | | Everone | HMDB | | Exten test | HMDB | | Malogen l.a. | HMDB | | Malogen l.a.200 | HMDB | | Orquisteron-e | HMDB | | Primotestone | HMDB | | reposo TMD | HMDB | | Testanthate | HMDB | | Testate | HMDB | | Testenate | HMDB | | Testinon | HMDB | | Testoenant | HMDB | | Testonenant | HMDB | | Testosterone 17beta-heptanoate | HMDB | | Testosterone 17beta-heptanoic acid | HMDB | | Testosterone enantate | HMDB | | Testosterone heptoate | HMDB | | Testosterone heptoic acid | HMDB | | Testosterone heptylate | HMDB | | Testosterone oenanthate | HMDB | | Testostroval | HMDB | | BTG Brand OF testosterone enanthate | MeSH, HMDB | | Jenapharm brand OF testosterone enanthate | MeSH, HMDB | | Rotexmedica brand OF testosterone enanthate | MeSH, HMDB | | Testosteron-depot jenapharm | MeSH, HMDB | | Pasadena brand OF testosterone enanthate | MeSH, HMDB | | Primoteston depot | MeSH, HMDB | | Rugby brand OF testosterone enanthate | MeSH, HMDB | | Testosteron depot-rotexmedica | MeSH, HMDB | | Testosteron-depot eifelfango | MeSH, HMDB | | Testrin p.a. | MeSH, HMDB | | Roberts brand OF testosterone enanthate | MeSH, HMDB | | eifelfango Brand OF testosterone enanthate | MeSH, HMDB | | Schering brand OF testosterone enanthate | MeSH, HMDB | | Theramed brand OF testosterone enanthate | MeSH, HMDB | | Theramex | MeSH, HMDB |
|
|---|
| Chemical Formula | C26H40O3 |
|---|
| Average Molecular Weight | 400.594 |
|---|
| Monoisotopic Molecular Weight | 400.297745146 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl heptanoate |
|---|
| Traditional Name | everone |
|---|
| CAS Registry Number | 315-37-7 |
|---|
| SMILES | [H][C@@]12CC[C@H](OC(=O)CCCCCC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C26H40O3/c1-4-5-6-7-8-24(28)29-23-12-11-21-20-10-9-18-17-19(27)13-15-25(18,2)22(20)14-16-26(21,23)3/h17,20-23H,4-16H2,1-3H3/t20-,21-,22-,23-,25-,26-/m0/s1 |
|---|
| InChI Key | VOCBWIIFXDYGNZ-IXKNJLPQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Androgen-skeleton
- Androstane-skeleton
- 3-oxosteroid
- 3-oxo-delta-4-steroid
- Oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Ketone
- Carboxylic acid ester
- Cyclic ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organooxygen compound
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 36 - 37.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-3479000000-622103246e9253c3578f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2594800000-2fe5b98ade2c6812e290 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-022i-5592000000-689f1cdb34f31cd98d15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-4490000000-d66a654ef39db2b12265 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0149000000-2bec5293e6f91f7dbff5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0393000000-1dfe91f6ac9835af8928 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-2190000000-387672165ae5773ca45b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0109000000-867f88cb280a910aabe9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01r2-1926000000-bc6e667c95b200d2ea91 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9101000000-86eb2f6d2ad8f02878cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0040900000-fe6a5cf79e52e56acc46 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-1981100000-256658d507dc1dea4517 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06r6-5920000000-9787c0613d8215ab200b | View in MoNA |
|---|
|
|---|