| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:18:32 UTC |
|---|
| Update Date | 2020-04-22 15:16:19 UTC |
|---|
| BMDB ID | BMDB0005789 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
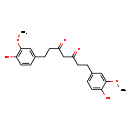
| Common Name | Tetrahydrocurcumin |
|---|
| Description | Tetrahydrocurcumin belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. Tetrahydrocurcumin exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on Tetrahydrocurcumin. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,7-Bis(4-hydroxy-3-methoxyphenyl)-3,5-heptanedione | ChEBI |
|
|---|
| Chemical Formula | C21H24O6 |
|---|
| Average Molecular Weight | 372.4117 |
|---|
| Monoisotopic Molecular Weight | 372.1572885 |
|---|
| IUPAC Name | 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione |
|---|
| Traditional Name | tetrahydrocurcumin |
|---|
| CAS Registry Number | 36062-04-1 |
|---|
| SMILES | COC1=CC(CCC(=O)CC(=O)CCC2=CC(OC)=C(O)C=C2)=CC=C1O |
|---|
| InChI Identifier | InChI=1S/C21H24O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h5-6,9-12,24-25H,3-4,7-8,13H2,1-2H3 |
|---|
| InChI Key | LBTVHXHERHESKG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Linear diarylheptanoids |
|---|
| Direct Parent | Curcuminoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Curcumin
- Gingerdione
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- 1,3-diketone
- Benzenoid
- 1,3-dicarbonyl compound
- Monocyclic benzene moiety
- Ketone
- Ether
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 95 - 97 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004r-0910000000-fa5f9c88947d04535eb3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0umr-9410140000-19c50b651dca5f38ec3c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0209000000-7dfd4c091aad667f4f26 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00bi-0922000000-4172923a1d3dfd5cefd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004r-1911000000-8ecd77e0bdc5eeb2914c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0109000000-cc999937febfc38f47c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0639000000-3347b9d7105791a849f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05ru-5986000000-5bfd0dc38ca2bb0b9efe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1049000000-140fb262cdbb389db98d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ks-6962000000-30302cd971363baba315 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3930000000-7ddc098840a945474666 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0219000000-9f478fd39c93a8ba2530 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0935000000-5253debe0acf5be8a731 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1921000000-1003e2c392e655b3af0f | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|