| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:18:19 UTC |
|---|
| Update Date | 2020-04-22 15:16:15 UTC |
|---|
| BMDB ID | BMDB0005774 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Endomorphin-2 |
|---|
| Description | Endomorphin-2 belongs to the class of organic compounds known as isoflavones. These are polycyclic compounds containing a 2-isoflavene skeleton which bears a ketone group at the C4 carbon atom. Based on a literature review a significant number of articles have been published on Endomorphin-2. |
|---|
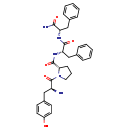
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Endomorphin 2 | HMDB | | H-Tyr-pro-phe-phe-NH2 | HMDB | | Tyrosyl-prolyl-phenylalanyl-phenylalaninamide | MeSH, HMDB | | Tyr-pro-phe-phe-NH2 | MeSH, HMDB | | (2S)-2-({[(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-N-[(1S)-1-(C-hydroxycarbonimidoyl)-2-phenylethyl]-3-phenylpropanimidate | Generator, HMDB | | Endomorphin-2 | MeSH | | L-Tyrosyl-L-prolyl-L-phenylalanyl-L-phenylalaninamide | MeSH, HMDB | | Tetrapeptide-15 | MeSH, HMDB |
|
|---|
| Chemical Formula | C32H37N5O5 |
|---|
| Average Molecular Weight | 571.6667 |
|---|
| Monoisotopic Molecular Weight | 571.279469319 |
|---|
| IUPAC Name | (2S)-2-{[(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]pyrrolidin-2-yl]formamido}-N-[(1S)-1-carbamoyl-2-phenylethyl]-3-phenylpropanamide |
|---|
| Traditional Name | (2S)-2-{[(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]pyrrolidin-2-yl]formamido}-N-[(1S)-1-carbamoyl-2-phenylethyl]-3-phenylpropanamide |
|---|
| CAS Registry Number | 141801-26-5 |
|---|
| SMILES | N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 |
|---|
| InChI Key | XIJHWXXXIMEHKW-LJWNLINESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoflavones. These are polycyclic compounds containing a 2-isoflavene skeleton which bears a ketone group at the C4 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Isoflav-2-enes |
|---|
| Direct Parent | Isoflavones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyisoflavonoid
- Isoflavone
- Chromone
- Benzopyran
- 1-benzopyran
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Pyranone
- Monocyclic benzene moiety
- Benzenoid
- Pyran
- Heteroaromatic compound
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 130 - 131 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01qi-7632920000-61c9c3747ad919a34c4d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0zfu-9443853000-b0f6cf2c32cb5ac7bcca | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Endomorphin-2,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0532290000-d0fd32e7d5d368aa05e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00yr-1942010000-5ab5f523355007c7035d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-5930000000-cd6666801968bdeee246 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0101190000-e9403040412cd1a9fc72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-2965550000-1bb3d8a636a7d6c56062 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-9741100000-f250d43c7be9e88c4b87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0200190000-588c4199c498359ce9ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abj-0900230000-da52f0e85cfb83709a36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-5900000000-8a102e76f50fa2d16995 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000190000-3c226367db9570ed339a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fu-5335790000-c4bd1a1b31a969d34acc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9683200000-5dde9bfa06e8110d9fc8 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Shimizu, Yoshiro; Takahashi, Motohiro; Fukumizu, Atsuko; Tsuda, Yuko; Bryant, Sharon D.; Lazarus, Lawrence H.; Okada, Yoshio. Synthesis of endomorphin analogs and studies on the structure-opioid receptor-binding activity relationship. Peptide Science (1999), Volume Date 1998, 35th 197-200. |
|---|