| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:20 UTC |
|---|
| Update Date | 2020-04-22 15:15:20 UTC |
|---|
| BMDB ID | BMDB0005085 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Leukotriene B4 dimethylamide |
|---|
| Description | Leukotriene B4 dimethylamide, also known as LTB4 dimethyl amide or LTB4DMA, belongs to the class of organic compounds known as n-acyl amines. N-acyl amines are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. Thus, leukotriene B4 dimethylamide is considered to be an eicosanoid. Based on a literature review very few articles have been published on Leukotriene B4 dimethylamide. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LTB4 Dimethyl amide | HMDB | | LTB4-Dimethylamide | HMDB | | N,N-Dimethyl-5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraen-1-amide | HMDB | | [S-[R*,s*-(e,Z,e,Z)]]-5,12-dihydroxy-N,N-dimethyl-6,8,10,14-eicosatetraenamide | HMDB | | LTB4dMA | HMDB | | Leukotriene b4 dimethylamide | MeSH |
|
|---|
| Chemical Formula | C22H37NO3 |
|---|
| Average Molecular Weight | 363.5341 |
|---|
| Monoisotopic Molecular Weight | 363.277344055 |
|---|
| IUPAC Name | (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxy-N,N-dimethylicosa-6,8,10,14-tetraenamide |
|---|
| Traditional Name | LTB4-dimethylamide |
|---|
| CAS Registry Number | 83024-92-4 |
|---|
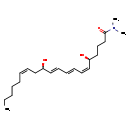
| SMILES | CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(=O)N(C)C |
|---|
| InChI Identifier | InChI=1S/C22H37NO3/c1-4-5-6-7-8-11-15-20(24)16-12-9-10-13-17-21(25)18-14-19-22(26)23(2)3/h8-13,16-17,20-21,24-25H,4-7,14-15,18-19H2,1-3H3/b10-9+,11-8-,16-12+,17-13-/t20-,21-/m1/s1 |
|---|
| InChI Key | BBJRTSLPWQUASB-UKODYPNASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl amines. N-acyl amines are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty amides |
|---|
| Direct Parent | N-acyl amines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-amine
- Tertiary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 3.493 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-6579000000-8c934e9462d2d7c37ac6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0006-9102600000-ee229477075ad4710074 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0009000000-998e72a31c1359378401 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gxt-5679000000-527bebcda5b12fba53f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9530000000-88c8cb32eeb988974040 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-8fb4339f3468eb35a70f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xu-1129000000-0e3ae91fe1d964d01498 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9241000000-5f90e31e4be94fdd9bfb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-5845818a3db050661528 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-1239000000-ec456dee5f3047301726 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007c-9274000000-1631deb55769407a4d34 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0119000000-33de9f29a7c0db4ef963 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fba-3229000000-3b7b404fcc3d15cfd804 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aos-9220000000-d48f0f349576a05fc4e0 | View in MoNA |
|---|
|
|---|