| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:15 UTC |
|---|
| Update Date | 2020-04-22 15:15:18 UTC |
|---|
| BMDB ID | BMDB0005081 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5-HEPE |
|---|
| Description | 5-HEPE, also known as (+-)-5-hepe, belongs to the class of organic compounds known as hydroxyeicosapentaenoic acids. These are eicosanoic acids with an attached hydroxyl group and five CC double bonds. Thus, 5-HEPE is considered to be an eicosanoid. Based on a literature review very few articles have been published on 5-HEPE. |
|---|
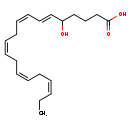
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-5-HEPE | ChEBI | | (+-)-5-Hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | ChEBI | | (+-)-5-Hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoate | Generator | | (+/-)-5-hepe | HMDB | | (+/-)-5-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoate | HMDB | | (+/-)-5-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | HMDB | | 5-Hydroxy-6,8,11,14,17-eicosapentaenoate | HMDB | | 5-Hydroxy-6,8,11,14,17-eicosapentaenoic acid | HMDB | | 5-Hydroxyeicosapentaenoate | HMDB | | 5-Hydroxyeicosapentaenoic acid | HMDB | | 5-HEPE | MeSH |
|
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Weight | 318.4504 |
|---|
| Monoisotopic Molecular Weight | 318.219494826 |
|---|
| IUPAC Name | (6E,8Z,11Z,14Z,17Z)-5-hydroxyicosa-6,8,11,14,17-pentaenoic acid |
|---|
| Traditional Name | 5-hydroxyeicosapentaenoic acid |
|---|
| CAS Registry Number | 92008-51-0 |
|---|
| SMILES | CC\C=C/C\C=C/C\C=C/C\C=C/C=C/C(O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19(21)17-15-18-20(22)23/h3-4,6-7,9-10,12-14,16,19,21H,2,5,8,11,15,17-18H2,1H3,(H,22,23)/b4-3-,7-6-,10-9-,13-12-,16-14+ |
|---|
| InChI Key | FTAGQROYQYQRHF-FCWZHQICSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyeicosapentaenoic acids. These are eicosanoic acids with an attached hydroxyl group and five CC double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Hydroxyeicosapentaenoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosapentaenoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-5394000000-3c896430e353b0ced0db | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0umj-8209200000-58c4694ad173b557f3c5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0149000000-dfe33b6babf270b34752 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udu-5693000000-fb5c896d30ace0c553d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-9880000000-5d7665b416fe42c2c371 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0069000000-023fef6c61163c7bc817 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-2093000000-e62551450dc40c949d52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9160000000-b435064b3bb6876f2d7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0019000000-816e1c2de0c22c09572d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-5298000000-38dbc1cf2b56e0e1e6ad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-9650000000-5f5552a443dffc55be3b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-1549000000-3a0bdd92ab4130f1a2cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-4932000000-a4abea64aa118c0599d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003u-9500000000-f8f2e81a65d2e71229cf | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|