| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:42 UTC |
|---|
| Update Date | 2020-05-11 20:24:31 UTC |
|---|
| BMDB ID | BMDB0005021 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
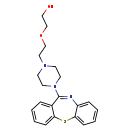
| Common Name | Quetiapine |
|---|
| Description | Quetiapine, also known as norsic or seroquel, belongs to the class of organic compounds known as dibenzothiazepines. Dibenzothiazepines are compounds containing a dibenzothiazepine moiety, which consists of two benzene connected by a thiazepine ring. Quetiapine is a very strong basic compound (based on its pKa). Quetiapine is a potentially toxic compound. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[2-(4-Dibenzo[b,F][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol | ChEBI | | Quetiapina | ChEBI | | Quetiapinum | ChEBI | | Norsic | Kegg | | Quetiapine fumarate | HMDB | | Seroquel | HMDB | | Ethanol, 2-(2-(4-dibenzo(b,F)(1,4)thiazepin-11-yl-1-piperazinyl)ethoxy)-, (e)-2-butenedioate (2:1) (salt) | HMDB | | Fumarate, quetiapine | HMDB | | 2-(2-(4-Dibenzo(b,F)(1,4)thiazepine-11-yl-1-piperazinyl)ethoxy)ethanol | HMDB |
|
|---|
| Chemical Formula | C21H25N3O2S |
|---|
| Average Molecular Weight | 383.507 |
|---|
| Monoisotopic Molecular Weight | 383.166747749 |
|---|
| IUPAC Name | 2-[2-(4-{2-thia-9-azatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,9,11,13-heptaen-10-yl}piperazin-1-yl)ethoxy]ethan-1-ol |
|---|
| Traditional Name | quetiapine |
|---|
| CAS Registry Number | 111974-69-7 |
|---|
| SMILES | OCCOCCN1CCN(CC1)C1=NC2=CC=CC=C2SC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C21H25N3O2S/c25-14-16-26-15-13-23-9-11-24(12-10-23)21-17-5-1-3-7-19(17)27-20-8-4-2-6-18(20)22-21/h1-8,25H,9-16H2 |
|---|
| InChI Key | URKOMYMAXPYINW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzothiazepines. Dibenzothiazepines are compounds containing a dibenzothiazepine moiety, which consists of two benzene connected by a thiazepine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazepines |
|---|
| Sub Class | Dibenzothiazepines |
|---|
| Direct Parent | Dibenzothiazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzothiazepine
- Diarylthioether
- Aryl thioether
- N-alkylpiperazine
- 1,4-diazinane
- Piperazine
- Benzenoid
- Imidolactam
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Carboxylic acid amidine
- Dialkyl ether
- Ether
- Amidine
- Organic 1,3-dipolar compound
- Thioether
- Propargyl-type 1,3-dipolar organic compound
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Organic nitrogen compound
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0007-9264000000-4758f7f7f4947282ccbb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fdo-9543200000-188eddba94dd2a57f908 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - DI-ESI-qTof , Negative | splash10-001i-0039000000-6e58caa07d6f7951f23e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001i-0089000000-b7b1c7d0bf6bb604c5cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0uk9-1390000000-ce855b6e08dee583681b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0ul0-0092000000-b8ad82fb58fb41b6f4fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0019000000-380bdfab40abc4fe9104 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00e9-2019000000-9a9f0ea1ddcfe3e51fba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-6942000000-82ff888287705b61f40d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1039000000-026ca51b770ab0fc287d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-2019000000-503cee9b3e7cc04ade6a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fu-9710000000-457979a8d0088bdeade2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-e07800842abb48579469 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000x-0019000000-f62857d5511d39f2c201 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-0191000000-5ce92b5e3a22486cc2f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1009000000-74ca90c52d883a3f4f25 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9027000000-059f5ab035332d4b8f68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p6-2192000000-1ea11b461430b2f762a1 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Bozsing, Daniel; Kovanyine, Lax Gyoergyi; Simig, Gyula; Rakoczy, Gyoergyne; Toempe, Peter; Krasznai, Gyoergy; Vereczkeyne, Donath Gyoergyi; Nagy, Kalman. A process for the preparation of quetiapine and its intermediates. 2001, Patent WO2001055125A1 (https://patents.google.com/patent/WO2001055125A1/en) |
|---|