| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:30 UTC |
|---|
| Update Date | 2020-05-11 19:58:21 UTC |
|---|
| BMDB ID | BMDB0005000 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Loratadine |
|---|
| Description | Loratadine, also known as claritin or alavert, belongs to the class of organic compounds known as benzocycloheptapyridines. These are aromatic compounds containing a benzene ring and a pyridine ring fused to a seven membered carbocycle. Based on a literature review a significant number of articles have been published on Loratadine. |
|---|
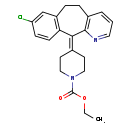
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylic acid ethyl ester | ChEBI | | Aerotina | ChEBI | | Alarin | ChEBI | | Alavert | ChEBI | | Alerpriv | ChEBI | | Allerclear | ChEBI | | Civeran | ChEBI | | Claratyne | ChEBI | | Claritin | ChEBI | | Loracert | ChEBI | | Loradamed | ChEBI | | Loradex | ChEBI | | Lorastine | ChEBI | | Loratadina | ChEBI | | Loratadinum | ChEBI | | Loratyne | ChEBI | | Wal-itin | ChEBI | | 4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylate ethyl ester | Generator | | 4-(8-Chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic acid ethyl ester | MeSH | | Clarium | MeSH | | Amantadine | HMDB | | Anhissen | HMDB | | Bonalerg | HMDB | | Claritine | HMDB | | Clarityn | HMDB | | Clarityne | HMDB | | Cronopen | HMDB | | Flonidan | HMDB | | Fristamin | HMDB | | Histaloran | HMDB | | Klaritin | HMDB | | Lertamine | HMDB | | Lisino | HMDB | | Loranox | HMDB | | Loratidine | HMDB | | Loratadine wyeth brand | MeSH, HMDB | | Wyeth brand OF loratadine | MeSH, HMDB |
|
|---|
| Chemical Formula | C22H23ClN2O2 |
|---|
| Average Molecular Weight | 382.883 |
|---|
| Monoisotopic Molecular Weight | 382.144805697 |
|---|
| IUPAC Name | ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate |
|---|
| Traditional Name | ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate |
|---|
| CAS Registry Number | 79794-75-5 |
|---|
| SMILES | CCOC(=O)N1CCC(CC1)=C1C2=C(CCC3=C1N=CC=C3)C=C(Cl)C=C2 |
|---|
| InChI Identifier | InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 |
|---|
| InChI Key | JCCNYMKQOSZNPW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzocycloheptapyridines. These are aromatic compounds containing a benzene ring and a pyridine ring fused to a seven membered carbocycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzocycloheptapyridines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzocycloheptapyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzocycloheptapyridine
- Piperidinecarboxylic acid
- Aryl chloride
- Aryl halide
- Piperidine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Carbamic acid ester
- Carbonic acid derivative
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 134 - 136 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 5.2 | SANGSTER (1994) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-6059000000-3d596b5ab5499c4089ca | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-001r-0039000000-928ed9c6b46ba83ebe20 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001r-1279000000-1c522c39e53b6bbfb9aa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001r-0039000000-928ed9c6b46ba83ebe20 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-067r-1292000000-3869907bd01f927b6f22 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-067i-0090000000-9bcbcadeda0bf07da702 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0frx-0090000000-2f8b7b5acca254e4cb20 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-067i-0090000000-b247e61957d933538341 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-dce8adc02434b224d589 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-7d2b8c3f5f87f99fcf7a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0api-0090000000-8419cf3b0c888e52c95b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-067r-0093000000-956a3618f6003ae45808 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-067i-0090000000-b62a29b4d6252b26f2bf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0009000000-d32917132c3359e65572 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0019000000-1acbfc6c05c2d600a966 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-001i-0009000000-4558440f48e82bf6eefb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0019-0009000000-ea1b61e9df959d6680c2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-067i-0090000000-cf7d75b43a3c2c0f0d1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-066r-0090000000-c5d1377bb7159732d4db | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-015i-0092000000-9bb7dcefe2f2f4f14bfd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-41dd9857a0206fa0f4e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ri-1029000000-0c5a179194107a35af3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015c-2091000000-fb50d1834a9a1d8d6c90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-1009000000-78af7865fc3439c125c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2009000000-df88de530c8ff41c69f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adl-7069000000-a5804bf835988dc83d2a | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|