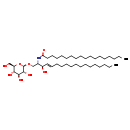| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:08 UTC |
|---|
| Update Date | 2020-05-11 20:54:19 UTC |
|---|
| BMDB ID | BMDB0004972 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Glucosylceramide (d18:1/18:0) |
|---|
| Description | Glucosylceramide (d18:1/18:0), also known as b-D-glucosyl-N-stearoylsphingosine or C18 glccer, belongs to the class of organic compounds known as glycosyl-n-acylsphingosines. Glycosyl-N-acylsphingosines are compounds containing a sphingosine linked to a simple glucosyl moiety. Glucosylceramide (d18:1/18:0) is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| beta-D-Glucosyl-(11)-N-octadecanoylsphing-4-enine | ChEBI | | beta-D-Glucosyl-N-stearoylsphingosine | ChEBI | | C18 GlcCer | ChEBI | | GlcCer(D18:1/18:0) | ChEBI | | N-(Octadecanoyl)-1-beta-glucosyl-sphing-4-enine | ChEBI | | b-D-Glucosyl-(11)-N-octadecanoylsphing-4-enine | Generator | | Β-D-glucosyl-(11)-N-octadecanoylsphing-4-enine | Generator | | b-D-Glucosyl-N-stearoylsphingosine | Generator | | Β-D-glucosyl-N-stearoylsphingosine | Generator | | N-(Octadecanoyl)-1-b-glucosyl-sphing-4-enine | Generator | | N-(Octadecanoyl)-1-β-glucosyl-sphing-4-enine | Generator | | b-D-Glucosyl-N-octadecanoylsphingosine | HMDB | | Β-D-glucosyl-N-octadecanoylsphingosine | HMDB | | 1-O-b-D-Glucopyranosyl-ceramide | HMDB | | 1-O-beta-delta-Glucopyranosyl-ceramide | HMDB | | Ganglioside GL1a | HMDB | | Gaucher cerebroside | HMDB | | GLC-beta1->1'cer | HMDB | | GlcCeramide | HMDB | | Glucocerebroside | HMDB | | Glucosylceramide | HMDB |
|
|---|
| Chemical Formula | C42H81NO8 |
|---|
| Average Molecular Weight | 728.0944 |
|---|
| Monoisotopic Molecular Weight | 727.596218573 |
|---|
| IUPAC Name | N-[(2S,3R,4E)-3-hydroxy-1-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}octadec-4-en-2-yl]octadecanamide |
|---|
| Traditional Name | N-[(2S,3R,4E)-3-hydroxy-1-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}octadec-4-en-2-yl]octadecanamide |
|---|
| CAS Registry Number | 85305-87-9 |
|---|
| SMILES | CCCCCCCCCCCCCCCCCC(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)[C@H](O)\C=C\CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C42H81NO8/c1-3-5-7-9-11-13-15-17-18-20-22-24-26-28-30-32-38(46)43-35(34-50-42-41(49)40(48)39(47)37(33-44)51-42)36(45)31-29-27-25-23-21-19-16-14-12-10-8-6-4-2/h29,31,35-37,39-42,44-45,47-49H,3-28,30,32-34H2,1-2H3,(H,43,46)/b31-29+/t35-,36+,37+,39+,40-,41+,42+/m0/s1 |
|---|
| InChI Key | YMYQEDCYNANIPI-DYJXBSQNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosyl-n-acylsphingosines. Glycosyl-N-acylsphingosines are compounds containing a sphingosine linked to a simple glucosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Glycosphingolipids |
|---|
| Direct Parent | Glycosyl-N-acylsphingosines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycosyl-n-acylsphingosine
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty amide
- Fatty acyl
- Monosaccharide
- N-acyl-amine
- Oxane
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Primary alcohol
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Endosome
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Insoluble | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0130342900-6ffc5f3d01ce03c04951 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-029w-0170392100-49f66daed67f6bab7d8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001c-0190113000-a73a590cd5f679d848a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1310041900-27fa50bb9f596bb3676f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08pi-5860790400-a33108b27bb384d72fdb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9442010000-b6435f233c20a9fdfd9b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ta-3000062900-5ef4db4827212a7ec310 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-5110193400-0c2eadd40ed34cfdb5a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9051020000-280774e07dab7928b2e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000900-a655397bdc68858249be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-5120136900-58b9f7cb5b3c0ecd8262 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0axr-9267120000-b3ceb137bfa88003084c | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | Not Available | View in JSpectraViewer |
|---|
|
|---|