| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:10:06 UTC |
|---|
| Update Date | 2020-04-22 15:13:59 UTC |
|---|
| BMDB ID | BMDB0004669 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 9-OxoODE |
|---|
| Description | 9-OxoODE, also known as 9-KODE or (10E,12Z)9-oxoode, belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. Thus, 9-oxoode is considered to be an octadecanoid. Based on a literature review a significant number of articles have been published on 9-OxoODE. |
|---|
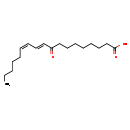
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (10E,12Z)-9-Oxooctadeca-10,12-dienoic acid | ChEBI | | (10E,12Z)9-oxo-ODE | ChEBI | | (10E,12Z)9-OxoODE | ChEBI | | 9-Keto-10E,12Z-octadecadienoic acid | ChEBI | | 9-KODE | ChEBI | | 9-oxo-10E,12Z-Octadecadienoic acid | ChEBI | | (10E,12Z)-9-Oxooctadeca-10,12-dienoate | Generator | | 9-Keto-10E,12Z-octadecadienoate | Generator | | 9-oxo-10E,12Z-Octadecadienoate | Generator | | 9-oxo-ODE | HMDB | | 9-OxoODE | ChEBI |
|
|---|
| Chemical Formula | C18H30O3 |
|---|
| Average Molecular Weight | 294.429 |
|---|
| Monoisotopic Molecular Weight | 294.219494826 |
|---|
| IUPAC Name | (10E,12Z)-9-oxooctadeca-10,12-dienoic acid |
|---|
| Traditional Name | 9-OxoODE |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCC\C=C/C=C/C(=O)CCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H30O3/c1-2-3-4-5-6-8-11-14-17(19)15-12-9-7-10-13-16-18(20)21/h6,8,11,14H,2-5,7,9-10,12-13,15-16H2,1H3,(H,20,21)/b8-6-,14-11+ |
|---|
| InChI Key | LUZSWWYKKLTDHU-ZJHFMPGASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- Long-chain fatty acid
- Unsaturated fatty acid
- Fatty acid
- Acryloyl-group
- Enone
- Alpha,beta-unsaturated ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9460000000-15e80ef34a1debfc0968 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0kmr-9542000000-0ccd4c1d848269239ab6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0190000000-60d81d12a11892c6999a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a7j-8980000000-a60d7dfde80f5bf722b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05nf-9310000000-09391ce48e072e4093fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-fa2d23e4158dbe12aafe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00tg-1790000000-63f9d414f1b592818328 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0c03-7910000000-5bf4d335642db628bb48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-e925eef3dc0f4d8cea02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0390000000-398c3f7d6e13ca3e756f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-5920000000-79b91d8ba73ffe3a7597 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-1290000000-605222a3edda7b9c4460 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a7i-8940000000-46e196ac4439dda35882 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0api-9200000000-a3f4eb735df6176bb3ff | View in MoNA |
|---|
|
|---|