| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:08:17 UTC |
|---|
| Update Date | 2020-04-22 15:13:26 UTC |
|---|
| BMDB ID | BMDB0004158 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
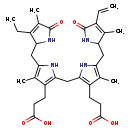
| Common Name | D-Urobilinogen |
|---|
| Description | D-D-d-urobilinogen belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. D-D-d-urobilinogen is possibly soluble (in water) and a moderately basic compound (based on its pKa). D-D-d-urobilinogen exists in all living species, ranging from bacteria to humans. In cattle, D-d-urobilinogen is involved in the metabolic pathway called the porphyrin metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Urobilinogen | HMDB | | Urobilinogen | HMDB |
|
|---|
| Chemical Formula | C33H42N4O6 |
|---|
| Average Molecular Weight | 590.7098 |
|---|
| Monoisotopic Molecular Weight | 590.310435096 |
|---|
| IUPAC Name | 3-(2-{[3-(2-carboxyethyl)-5-[(4-ethenyl-3-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-2-yl]methyl}-5-[(3-ethyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| Traditional Name | 3-(2-{[3-(2-carboxyethyl)-5-[(4-ethenyl-3-methyl-5-oxo-1,2-dihydropyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-2-yl]methyl}-5-[(3-ethyl-4-methyl-5-oxo-1,2-dihydropyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| CAS Registry Number | 17208-65-0 |
|---|
| SMILES | CCC1=C(C)C(=O)NC1CC1=C(C)C(CCC(O)=O)=C(CC2=C(CCC(O)=O)C(C)=C(CC3NC(=O)C(C=C)=C3C)N2)N1 |
|---|
| InChI Identifier | InChI=1S/C33H42N4O6/c1-7-20-19(6)32(42)37-27(20)14-25-18(5)23(10-12-31(40)41)29(35-25)15-28-22(9-11-30(38)39)17(4)24(34-28)13-26-16(3)21(8-2)33(43)36-26/h8,26-27,34-35H,2,7,9-15H2,1,3-6H3,(H,36,43)(H,37,42)(H,38,39)(H,40,41) |
|---|
| InChI Key | KSQFFJKKJAEKTB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Pyrroline
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-5600190000-39b380b30d3296452a8a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-7600019000-726abed506367aa18efc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0009110000-22201ddfa15f4e8ab99f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000090000-e7d752494982063db039 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0200290000-a50512ce586c48b2dd77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-2000920000-82cae0801f5ae06ee29d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-0000090000-c473bc91332a49995cbb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ds-1100090000-63230997999368b5e280 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9010220000-1e3be1cd0f823364b481 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0000960000-020da40faedc9ba305fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0210930000-047750634ae90193f501 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001j-3712920000-c83ff79b31097b8c2289 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000290000-464cdd1459737f902444 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0k9l-2310790000-058bc692c3cc849a4879 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0076-3970320000-c5bd97c9063dcb386cfd | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|