| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:07:51 UTC |
|---|
| Update Date | 2020-04-22 15:13:17 UTC |
|---|
| BMDB ID | BMDB0004087 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
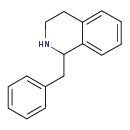
| Common Name | 1-Benzyl-1,2,3,4-tetrahydroisoquinoline |
|---|
| Description | 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, also known as (R,S)-tetrahydrobenzylisoquinoline or 1BNTIQ, belongs to the class of organic compounds known as benzylisoquinolines. These are organic compounds containing an isoquinoline to which a benzyl group is attached. Based on a literature review a significant number of articles have been published on 1-Benzyl-1,2,3,4-tetrahydroisoquinoline. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R,S)-Tetrahydrobenzylisoquinoline | ChEBI | | 1,2,3,4-Tetrahydro-1-(phenylmethyl)isoquinoline | ChEBI | | 1BnTIQ | ChEBI | | (RS)-1-Benzyl-1,2,3,4-tetrahydroisoquinoline | Kegg | | 1,2,3,4-tetrahydro-1-(Phenylmethyl)isoquinoline, (+-)-isomer | MeSH, HMDB | | 1,2,3,4-tetrahydro-1-(Phenylmethyl)isoquinoline, (R)-isomer | MeSH, HMDB | | 1,2,3,4-tetrahydro-1-(Phenylmethyl)isoquinoline, (S)-isomer | MeSH, HMDB | | 1,2,3,4-tetrahydro-1-(Phenylmethyl)isoquinoline hydrochloride | MeSH, HMDB | | S49 Isoquinoline | MeSH, HMDB | | 1-Benzyl-1,2,3,4-tetrahydroisoquinoline | MeSH |
|
|---|
| Chemical Formula | C16H17N |
|---|
| Average Molecular Weight | 223.3129 |
|---|
| Monoisotopic Molecular Weight | 223.136099549 |
|---|
| IUPAC Name | 1-benzyl-1,2,3,4-tetrahydroisoquinoline |
|---|
| Traditional Name | 1BnTIQ |
|---|
| CAS Registry Number | 19716-56-4 |
|---|
| SMILES | C(C1NCCC2=CC=CC=C12)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C16H17N/c1-2-6-13(7-3-1)12-16-15-9-5-4-8-14(15)10-11-17-16/h1-9,16-17H,10-12H2 |
|---|
| InChI Key | YRYCIFUZSUMAAY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzylisoquinolines. These are organic compounds containing an isoquinoline to which a benzyl group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoquinolines and derivatives |
|---|
| Sub Class | Benzylisoquinolines |
|---|
| Direct Parent | Benzylisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylisoquinoline
- Tetrahydroisoquinoline
- Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- Azacycle
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-2900000000-69767d6f6a91a5c24bd1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-75679a6f444b9bd42db4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05gl-3890000000-5df7dde54dbe0fe2b4fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9700000000-3f687e462c9cd0cc1ff2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-672b95459dab89c79e3c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-87a0dda1779b4676ba7c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-1910000000-d2ecf13f470a7ba9cb0e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-279452a29859034561fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-939f38e3140d9ca30df6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-005c-2900000000-ed789626d72de391b664 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-0e93eae718d771100a69 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-2960000000-a0da7d0090f88d305999 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4m-4900000000-148ae7c1169e2d0c08f0 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Shinohara, Tatsumi; Takeda, Akira; Toda, Jun; Terasawa, Noriyo; Sano, Takehiro. A highly efficient synthesis of 1-methyl-, 1-benzyl-, and 1-phenyl-1,2,3,4-tetrahydroisoquinolines by a modified Pummerer reaction. Heterocycles (1997), 46 555-566. |
|---|