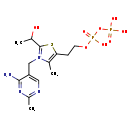| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:06:32 UTC |
|---|
| Update Date | 2020-04-22 15:12:53 UTC |
|---|
| BMDB ID | BMDB0003904 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-(a-Hydroxyethyl)thiamine diphosphate |
|---|
| Description | 2-(a-Hydroxyethyl)thiamine diphosphate, also known as 2-hydroxyethyl-THPP, belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. 2-(a-Hydroxyethyl)thiamine diphosphate exists in all living species, ranging from bacteria to plants to humans. Based on a literature review very few articles have been published on 2-(a-Hydroxyethyl)thiamine diphosphate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(alpha-Hydroxyethyl)thiamine diphosphate | ChEBI | | 2-Hydroxyethyl-THPP | ChEBI | | 2-(a-Hydroxyethyl)thiamine diphosphoric acid | Generator | | 2-(alpha-Hydroxyethyl)thiamine diphosphoric acid | Generator | | 2-(Α-hydroxyethyl)thiamine diphosphate | Generator | | 2-(Α-hydroxyethyl)thiamine diphosphoric acid | Generator | | 3-[(4-amino-2-Methyl-5-pyrimidinyl)methyl]-2-(1-hydroxyethyl)-4-methyl-5-(4,6,6-trihydroxy-3,5-dioxa-4,6-diphosphahex-1-yl)- P,p'-dioxide | HMDB | | 3-[(4-amino-2-Methyl-5-pyrimidinyl)methyl]-2-(1-hydroxyethyl)-5-(2-hydroxyethyl)-4-methyl- 5-(trihydrogen pyrophosphate) (8ci) | HMDB | | Thiazolium | HMDB |
|
|---|
| Chemical Formula | C14H23N4O8P2S |
|---|
| Average Molecular Weight | 469.367 |
|---|
| Monoisotopic Molecular Weight | 469.071182446 |
|---|
| IUPAC Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-2-(1-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional Name | 2-Hydroxyethyl-ThPP |
|---|
| CAS Registry Number | 10055-47-7 |
|---|
| SMILES | CC(O)C1=[N+](CC2=CN=C(C)N=C2N)C(C)=C(CCOP(O)(=O)OP(O)(O)=O)S1 |
|---|
| InChI Identifier | InChI=1S/C14H22N4O8P2S/c1-8-12(4-5-25-28(23,24)26-27(20,21)22)29-14(9(2)19)18(8)7-11-6-16-10(3)17-13(11)15/h6,9,19H,4-5,7H2,1-3H3,(H4-,15,16,17,20,21,22,23,24)/p+1 |
|---|
| InChI Key | RRUVJGASJONMDY-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 2,4,5-trisubstituted 1,3-thiazole
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Heteroaromatic compound
- Azole
- Thiazole
- Secondary alcohol
- Azacycle
- Hydrocarbon derivative
- Amine
- Organic oxide
- Primary amine
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic alcohol
- Organic oxygen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 0.401 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004j-7935300000-0b34a7fca682f8a54c08 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004j-6902440000-140c534b7a34d2b16ee4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0010900000-edc3bde291066610b257 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-6105900000-00a7fd6e1e5ff7ec29f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-8890500000-4cdd741793f8ee1a2bec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-7931ef394e437f66ed47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9100200000-d75312b43c4b5a131db6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00bj-9000000000-6e1658898bc7c721e474 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xr-0603900000-82b7c28c4be6ddfaa3f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0907300000-f357687aa15a122efae0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2900000000-4e0cf5cd46cb10b9f12f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|