| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:05:37 UTC |
|---|
| Update Date | 2020-04-22 15:12:36 UTC |
|---|
| BMDB ID | BMDB0003633 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
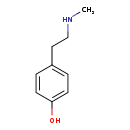
| Common Name | N-Methyltyramine |
|---|
| Description | N-Methyltyramine, also known as NMT, belongs to the class of organic compounds known as phenethylamines. Phenethylamines are compounds containing a phenethylamine moiety, which consists of a phenyl group substituted at the second position by an ethan-1-amine. N-Methyltyramine is a very strong basic compound (based on its pKa). N-Methyltyramine exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-N-methylphenethylamine | ChEBI | | Methyl-4-tyramine | ChEBI | | p-(2-Methylaminoethyl)phenol | ChEBI | | 4-(2-(Methylamino)ethyl)-phenol | HMDB | | 4-(2-Methylaminoethyl)phenol | HMDB | | 4-[2-(Methylamino)ethyl]-phenol | HMDB | | 4-[2-(Methylamino)ethyl]phenol | HMDB | | Methyl tyramine | HMDB | | N-Methyl-p-tyramine | HMDB | | N-Methyltyraminium | HMDB | | NMT | HMDB | | p-(2-(Methylamino)ethyl)phenol | HMDB | | p-[2-(Methylamino)ethyl]-phenol | HMDB | | {p-[2-(methylamino)ethyl]phenol} | HMDB | | N-Methyltyrosamine | HMDB | | Methyl-4-tyramine hydrochloride | HMDB | | Methyl-4-tyramine hydrobromide | HMDB |
|
|---|
| Chemical Formula | C9H13NO |
|---|
| Average Molecular Weight | 151.2056 |
|---|
| Monoisotopic Molecular Weight | 151.099714043 |
|---|
| IUPAC Name | 4-[2-(methylamino)ethyl]phenol |
|---|
| Traditional Name | N-methyltyramine |
|---|
| CAS Registry Number | 370-98-9 |
|---|
| SMILES | CNCCC1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13NO/c1-10-7-6-8-2-4-9(11)5-3-8/h2-5,10-11H,6-7H2,1H3 |
|---|
| InChI Key | AXVZFRBSCNEKPQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenethylamines. Phenethylamines are compounds containing a phenethylamine moiety, which consists of a phenyl group substituted at the second position by an ethan-1-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Phenethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenethylamine
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Phenol
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.455 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-14293e75666be4e27019 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00xr-7920000000-4709651ba3c22c23b463 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0900000000-0f335d13104342fdeed5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-2900000000-037595c7cc0c504058c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kk9-9500000000-376efc98f806aac30929 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-27ea78b166d8b3172ce1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1900000000-fb693eca1267a0ba9685 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05al-6900000000-6d964137e90c9e3293d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1900000000-6ac9e0419aa31060260b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-7900000000-7fd4cbc25b6ff2a2bf1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-9100000000-7021336d37b527e2e5fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-1c71ef4ed8c1b09fb863 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-4f21e6ca90b01b880bf4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9600000000-4a0119f5c8223bec2ae4 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-89ff5a43bc3e7737bcb6 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|