| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:05:08 UTC |
|---|
| Update Date | 2020-05-11 20:34:44 UTC |
|---|
| BMDB ID | BMDB0003539 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
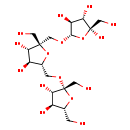
| Common Name | Levan |
|---|
| Description | Levan, also known as polyfructose, belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. Based on a literature review very few articles have been published on Levan. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Polyfructose | MeSH | | (2,6-beta-D-Fructosyl)N | HMDB | | (2,6-beta-D-Fructosyl)n+1 | HMDB | | (2,6-beta-delta-Fructosyl)N | HMDB | | (2,6-beta-delta-Fructosyl)n+1 | HMDB | | 2,6-beta-D-Fructan | HMDB | | 2,6-beta-delta-Fructan | HMDB | | Fructan | HMDB | | Levan N | HMDB |
|
|---|
| Chemical Formula | C18H32O16 |
|---|
| Average Molecular Weight | 504.4371 |
|---|
| Monoisotopic Molecular Weight | 504.169034976 |
|---|
| IUPAC Name | (2R,3S,4S,5S)-5-{[(2S,3S,4S,5R)-5-({[(2R,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}methyl)-3,4-dihydroxy-2-(hydroxymethyl)oxolan-2-yl]methoxy}-2-(hydroxymethyl)oxolane-2,3,4-triol |
|---|
| Traditional Name | fructans |
|---|
| CAS Registry Number | 9013-95-0 |
|---|
| SMILES | OC[C@H]1O[C@@](CO)(OC[C@H]2O[C@@](CO)(CO[C@H]3O[C@](O)(CO)[C@@H](O)[C@@H]3O)[C@@H](O)[C@@H]2O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C18H32O16/c19-1-7-9(23)14(28)18(5-22,33-7)31-2-8-10(24)12(26)16(3-20,32-8)6-30-15-11(25)13(27)17(29,4-21)34-15/h7-15,19-29H,1-6H2/t7-,8-,9-,10-,11+,12+,13+,14+,15+,16+,17-,18-/m1/s1 |
|---|
| InChI Key | AIHDCSAXVMAMJH-GFBKWZILSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Disaccharide
- O-glycosyl compound
- Ketal
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Polyol
- Organoheterocyclic compound
- Ether
- Oxacycle
- Dialkyl ether
- Acetal
- Primary alcohol
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-6240900000-f1c5bb82595b7adde7a0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0159-4403119000-0804d302073c73682666 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05n0-2689750000-eb151ecc90899c68eb42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-0709000000-943bc21e712c718fe599 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uec-4920000000-89d6f0013703f6c7c921 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-3904110000-2e605b3697e7dbace613 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ta-0900100000-3bc5ee3e1276649fddf1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002g-6900000000-d203a8851f7ebaeeb171 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0504980000-ec443f82f8215c5498e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0550-5918320000-d87ea24b93941a8934e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07i6-9331000000-4379b9b8f4faa92df1ba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-0109780000-e8a164594ecf7a12328d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ab9-9735610000-17245480c4f31d107b11 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9301000000-8242465d27bd00a06bd3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Park, Hae-Eun; Park, Na Hee; Kim, Min-Jeong; Lee, Tae Ho; Lee, Hyeon Gyu; Yang, Ji-Young; Cha, Jaeho. Enzymatic synthesis of fructosyl oligosaccharides by levansucrase from Microbacterium laevaniformans ATCC 15953. Enzyme and Microbial Technology (2003), 32(7) p.820-827 |
|---|