| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:34 UTC |
|---|
| Update Date | 2020-04-22 15:11:58 UTC |
|---|
| BMDB ID | BMDB0003323 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Biotripyrrin-a |
|---|
| Description | Biotripyrrin-a belongs to the class of organic compounds known as substituted pyrroles. These are heterocyclic compounds containing a pyrrole ring substituted at one or more positions. Based on a literature review a small amount of articles have been published on Biotripyrrin-a. |
|---|
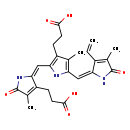
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (Z,Z)-2-[[3-(2-Carboxyethyl)-5-[(3-ethenyl-1,5-dihydro-4-methyl-5-oxo-2H-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-2-yl]methylene]-2,5-dihydro-4-methyl-5-oxo-1H-pyrrole-3-propanoate | HMDB | | (Z,Z)-2-[[3-(2-Carboxyethyl)-5-[(3-ethenyl-1,5-dihydro-4-methyl-5-oxo-2H-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-2-yl]methylene]-2,5-dihydro-4-methyl-5-oxo-1H-pyrrole-3-propanoic acid | HMDB | | 1,14,15,17-Tetrahydro-2,7,13-trimethyl-1,14- dioxo-3-vinyl-16H-tripyrrin-8,12-dipropionate | HMDB | | 1,14,15,17-Tetrahydro-2,7,13-trimethyl-1,14- dioxo-3-vinyl-16H-tripyrrin-8,12-dipropionic acid | HMDB | | Biotripyrrin a | HMDB | | 3-(2-{[(2E)-3-(2-carboxyethyl)-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-5-{[(2E)-3-ethenyl-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoate | HMDB | | Biotripyrrin-a | MeSH |
|
|---|
| Chemical Formula | C25H27N3O6 |
|---|
| Average Molecular Weight | 465.4984 |
|---|
| Monoisotopic Molecular Weight | 465.189985611 |
|---|
| IUPAC Name | 3-[(2E)-2-{[3-(2-carboxyethyl)-5-{[(2E)-3-ethenyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methylidene}-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-3-yl]propanoic acid |
|---|
| Traditional Name | 3-[(2E)-2-{[3-(2-carboxyethyl)-5-{[(2E)-3-ethenyl-4-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methylidene}-4-methyl-5-oxo-1H-pyrrol-3-yl]propanoic acid |
|---|
| CAS Registry Number | 158649-79-7 |
|---|
| SMILES | CC1=C(CCC(O)=O)\C(NC1=O)=C/C1=C(CCC(O)=O)C(C)=C(N1)\C=C1\NC(=O)C(C)=C1C=C |
|---|
| InChI Identifier | InChI=1S/C25H27N3O6/c1-5-15-13(3)24(33)27-19(15)10-18-12(2)16(6-8-22(29)30)20(26-18)11-21-17(7-9-23(31)32)14(4)25(34)28-21/h5,10-11,26H,1,6-9H2,2-4H3,(H,27,33)(H,28,34)(H,29,30)(H,31,32)/b19-10+,21-11+ |
|---|
| InChI Key | DXWHHYOVLWSVQD-WOEXBRBNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as substituted pyrroles. These are heterocyclic compounds containing a pyrrole ring substituted at one or more positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrroles |
|---|
| Sub Class | Substituted pyrroles |
|---|
| Direct Parent | Substituted pyrroles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrroline
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0aba-0101900000-576e37d4533cbaafeb47 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-009f-6000290000-67f24cfb96a315504935 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-fdb73ab1676150443d73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fs-0104900000-ab61c92fb1bcd3a2ba6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-032l-2947100000-3626428d6f659f777ca9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-0000900000-a4a992672d99c12f01ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-1000900000-70df34b8e40d06f126ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000200000-187676c490102674ea7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0100900000-b9f4281a345dffaac87e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0005900000-e7a99e8231c597ee35bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0209100000-f38d93a1840788367b32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r2-0000900000-9dc85a74c1e43c202961 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0007900000-4318749e4cfd75578f58 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fmr-0129300000-3f071be07f1f3a5ee0f8 | View in MoNA |
|---|
|
|---|