| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:33 UTC |
|---|
| Update Date | 2020-04-22 15:11:58 UTC |
|---|
| BMDB ID | BMDB0003318 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
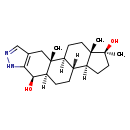
| Common Name | 4b-Hydroxystanozolol |
|---|
| Description | 4b-Hydroxystanozolol belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. Based on a literature review a small amount of articles have been published on 4b-Hydroxystanozolol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4b,5a,17b)-17-Methyl-2'H-androst-2-eno[3,2-c]pyrazole-4,17-diol | HMDB | | 4b-OH-Stanozolol | HMDB | | 4beta-Hydroxystanozolol | HMDB | | Cyclopenta[7,8]phenanthro[2,3-c]pyrazole 2'H-androst-2-eno[3,2-c]pyrazole-4,17-diol deriv. | HMDB |
|
|---|
| Chemical Formula | C21H32N2O2 |
|---|
| Average Molecular Weight | 344.491 |
|---|
| Monoisotopic Molecular Weight | 344.246378278 |
|---|
| IUPAC Name | (1S,2R,9R,10R,13R,14S,17S,18S)-2,17,18-trimethyl-6,7-diazapentacyclo[11.7.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁸]icosa-4(8),5-diene-9,17-diol |
|---|
| Traditional Name | (1S,2R,9R,10R,13R,14S,17S,18S)-2,17,18-trimethyl-6,7-diazapentacyclo[11.7.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁸]icosa-4(8),5-diene-9,17-diol |
|---|
| CAS Registry Number | 125636-92-2 |
|---|
| SMILES | [H][C@@]12CC[C@](C)(O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])[C@@H](O)C3=C(C[C@]12C)C=NN3 |
|---|
| InChI Identifier | InChI=1S/C21H32N2O2/c1-19-10-12-11-22-23-17(12)18(24)16(19)5-4-13-14(19)6-8-20(2)15(13)7-9-21(20,3)25/h11,13-16,18,24-25H,4-10H2,1-3H3,(H,22,23)/t13-,14+,15+,16+,18-,19-,20+,21+/m1/s1 |
|---|
| InChI Key | OCUSYXNRARMJHS-SUVJOWDWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrane-skeleton
- 4-hydroxysteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Azole
- Cyclic alcohol
- Pyrazole
- Tertiary alcohol
- Heteroaromatic compound
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00or-0359000000-0f134279c73ede2dfb19 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-2013900000-570ee78d58d7e1f57eeb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-072017f9a5446fbf31a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-2079000000-cc0727df2fc91ce42e51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-9513000000-d38b2a43ed8ef01cf863 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-c5c4fa8fa856784dd5f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ov-0019000000-b88ee2c0dfea5cb32728 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2049000000-33814ebb11889f38fe27 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-f0c9f6f6d6de10398e83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0009000000-b6d1574e79022e9c9f59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-0019000000-24be4b8b2ce190c4242a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0019000000-a9ee0abfecf2da386d7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-2794000000-ea9b314b90ba7e8c6771 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-1290000000-2f1310462383fa6e8904 | View in MoNA |
|---|
|
|---|